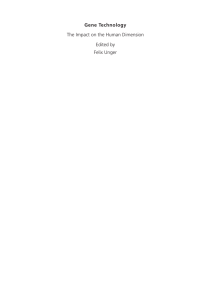
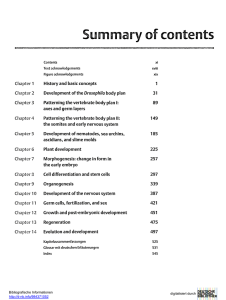
Animal Reproduction Science 164 (2016) 9–13 Contents lists available at ScienceDirect Animal Reproduction Science journal homepage: www.elsevier.com/locate/anireprosci Sex determination of ovine embryos by SRY and amelogenin (AMEL) genes using maternal circulating cell free DNA Adel Saberivand a,∗ , Sima Ahsan b a b Division of Theriogenology, Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran Graduate in Veterinary Medicine, Urmia University, Urmia, Iran a r t i c l e i n f o Article history: Received 27 December 2014 Received in revised form 23 September 2015 Accepted 30 October 2015 Available online 1 November 2015 Keywords: Sex determination Amelogenin SRY Embryos Ghezel Sheep PCR a b s t r a c t Simple and precise methods for sex determination in animals are a pre-requisite for a number of applications in animal production and forensics. Some of the existing methods depend only on the detection of Y-chromosome specific sequences. However, the detection of Y and X-chromosome specific sequences is advantageous. In the present study the accuracy of sex determination by SRY (sex-determining region Y) and AMEL (Amelogenin) gene detection was assessed using a polymerase chain reaction (PCR) of DNA extracted from free fetal cells in maternal blood, which is noninvasive for fetus and easier to collect. The PCR amplification of SRY primers produced a single band of 171 bp from ewes bearing a male fetus, whereas no band was amplified from the DNA extracted from ewes pregnant to a female fetus. Moreover, two bands of 182 and 242 bp in male and a single band of 242 in female fetuses were produced by AMEL gene primers in the PCR reaction. Using this technique 100% of samples were successfully sexed, excluding twins. In conclusion, we demonstrated that sex determination using DNA of free fetal cells in maternal plasma is efficient using both SRY and AMEL gene sequences. It also is evident that this method is not suitable for sex determination of twin pregnancies. © 2015 Elsevier B.V. All rights reserved. 1. Introduction One of the basic and important elements of animal production is scientific progress on reproductive technologies (Dervishi et al., 2011). On the other hand, fetal sex detection is a key practice in livestock management. It is extremely important in ovine industry to identify lamb gender for breeding, culling and eliminating expenses of the progeny test programs (Kadivar et al., 2013). ∗ Corresponding author at: Division of Theriogenology, Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Tabriz, 29 Bahman Blvd., Tabriz, Iran. E-mail addresses: [email protected], [email protected] (A. Saberivand). http://dx.doi.org/10.1016/j.anireprosci.2015.10.011 0378-4320/© 2015 Elsevier B.V. All rights reserved. Several different methods have been used to determine mammalian prenatal sex. These methods are: trans-rectal ultrasonography (de Freitas Neto et al., 2010), cytogenetic analysis (King, 1984), detection of H-Y antigen (Watchel, 1984), measurement of X-linked enzymes before Barr body formation (Kageyama et al., 2004). The need to harvest cells in metaphase is a limitation of cytological detection. In situ hybridization and fluorescent in situ hybridization (FISH) is time consuming (Dervishi et al., 2011) and the success of trans-rectal ultrasonography is depended on the fetal position, age and the operator’s experience (Ali, 2004). Although the presence of fetal nucleic acids in the maternal circulation was discovered decades ago (Mandel and Métais, 1948), circulating cell free fetal DNA (ccffDNA), was just identified in maternal plasma in late 1990s (Lo et al., 1997) when they correctly identified male fetuses in 80% of the pregnant women carrying male fetus. This 10 A. Saberivand, S. Ahsan / Animal Reproduction Science 164 (2016) 9–13 revolutionary-like event has emphasized that the placenta is no longer thought to be an impermeable membrane (Swarup and Rajeswari, 2007) and provided new approach for prenatal molecular diagnosis such as fetal sex determination, screening for pregnancy-related complications and fetal diseases (Lo et al., 1998; Jimenez and Tarantal, 2003; Maron et al., 2007). Embryonic sex determination using sex-specific DNA sequences by Polymerase chain reaction (PCR) has been used in cattle (Peura et al., 1991; da Cruz et al., 2012), pigs (Fajfar-Whetstone et al., 1993), humans (Handyside et al., 1989) and mice (Kunieda et al., 1992). Because of acceptable reliability, high sensitivity, inexpensiveness and rapidity, PCR is currently used as genotypic sex determination method (Dervishi et al., 2011). The main drawback of this method is that the absence of amplification is interpreted as the fetus being female. To overcome this issue, a gene which is present in male and female should be amplified at the same time. Amplification of ZFX/Y region and Restriction Fragment Length Polymorphism analysis (Aasen and Medrano, 1990) and Duplex PCR co-amplifying a specific Y-chromosomal sequence and an autosomal sequence as a control have been used (Appao Rat and Totey, 1999; Mara et al., 2007). Another issue is the type of ovine placenta. Unlike the humans (hemochorial), sheep has a synepitheliochorial placenta which does not permit a direct contact between the maternal blood and fetal trophoblast (Wooding, 1992). Therefore, the likelihood of the passage of fetal DNA to maternal circulation is very scarce, highlighting the importance of the sensitivity of DNA extraction methods to be used. It is the presence or absence of the Y chromosome determines the sex of fetus during sexual differentiation. Therefore, the sex determining region, SRY has been the most frequent gene used for sex typing (Piprek, 2010), and seems to have greatest homology within the ruminant species (Pomp et al., 1995). The amelogenin (AMEL) gene, which exists on both X (AMELX) and Y (AMELY) chromosomes, has been used to determine the sex in cattle (Ennis and Gallager, 1994), sheep and deer (Pfeiffer and Brenig, 2005; Dervishi et al., 2011; Kadivar et al., 2013). The aim of the present study was to use cell free fetal DNA in pregnant ewe plasma to determine fetal sex in a PCR assay using SRY and amelogenin (AMELX/AMELY) genes. 2. Material and methods 2.1. Blood collection and plasma harvesting Fifty one mature Ghezel ewes in gestational weeks of 8–20 from private sector and from animals brought to government-owned clinics were used in this study. In addition, three normal non-pregnant ewes and three normal rams were used as control animals. As a source of ccffDNA, peripheral blood samples were obtained from the animals. A volume of 5 mL of blood samples was collected from jugular vein into the EDTA vacutainer tubes and centrifuged at 1600 × g at room temperature for 10 min to separate plasma from packed cells and buffy coat. Subsequently, they were centrifuged at 16,000 × g for 10 min to further separate cellular debris. The blood plasma samples were stored at −20 ◦ C until analysis. 2.2. DNA extraction from maternal blood plasma DNA was extracted from 100 l plasma using a commercial DNA Purification kit (Sinaclon, Iran) as recommended by the manufacturer. The total DNA extracted from the cells was used as template in PCR for sex determination. The quality and quantity of DNA were determined using Biophotometer (Eppendorf, Germany). The primers were synthesized (Sinagen, Iran) for SRY and AMEL according to Dervishi et al. (2011) and were as follows; SRY gene; upstream: 5 - GACAATCATAGCGCAAACGA-3 , downstream: 5 -CAGCTGCTTGCTGATCTCTG-3 ; AMEL gene; upstream: 5 -CCGCCCAGCAGCCCTTCC-3 , downstream: 5 -CCCGCTTGGTCTTGTCTGTTGC-3 . Amplification conditions were identical for two genes except the thermal profile as is stated. The amplification reactions were set in a final volume of 25 l containing 5 pmol of each primer, 200 mM of each dNTPs, 2 mM MgCL2 , 50 mM KCL, 10 mM Tris–HCL, 0.5 U Taq DNA Polymerase and 100 ng of genomic DNA. The thermal profile was as follows; initial denaturation at 94 ◦ C for 5 min. Thirty-five cycles with the following step-cycle profile: denaturation at 94 ◦ C for 45 s, followed by primer annealing at 63 ◦ C (for AMEL) and 55 ◦ C (for SRY) for 45 s, and primer extension at 72 ◦ C for 45 s. The last extension step was 10 min longer. An aliquot of each reaction mixture was subjected to electrophoresis in 2% agarose gel and stained with safe stain (Sinaclone, Iran). The PCRbased sex of embryos were compared with the phenotypic sex and presented as percentage data. Efficiency of the method was evaluated in percentage (%) for both genes. 3. Results Fetal male (SRY and AMELY) and female (AMELX) circulating DNA was successfully indicated in the maternal plasma in the gravid ewes. Out of 51 samples, 8 samples (15.68%) were twins that were not used in the analysis. For comparison, one sample of twins was used and analyzed (data are not included). The stage of pregnancy and PCR detected sex by PCR for both SRY and AMEL genes are summarized in Table 1. There was no significant difference between stages of pregnancy for both genes determining fetal sex. The PCR amplification of SRY primers on DNA extracted from blood plasma of ewes bearing a male fetus produced a single band of 171 bp, whereas no band was amplified from the DNA extracted from ewes pregnant to a female fetus (Fig. 1). Twenty one out of 23 (91.30%) female fetuses were correctly identified by SRY gene sex determination. The number of male fetuses identified by this gene was 19 out of 20 (95%). The overall test accuracy for correct sex determination for SRY gene was 93.15%. Two bands of 182 and 242 bp in male and a single band of 242 in female fetuses were produced by AMEL gene primers in the PCR reaction (Fig. 2). Twenty two out of 23 (95.65%) A. Saberivand, S. Ahsan / Animal Reproduction Science 164 (2016) 9–13 11 Fig. 1. PCR products of SRY gene from pregnant ewe plasma samples. Lane 1: negative control, lane 2: positive control, lanes 4, 5 and 6 ewes with male fetuses. Lanes 3 and 6, ewes with female fetuses. Lane 8, 100 bp DNA marker. Fig. 2. PCR products of AMEL gene from pregnant ewe plasma samples. Lane 1: 100 bp DNA marker. negative control, lane 2: positive control female ewe, lane 3: positive control male ewe, lanes 4 and 7 ewes with female fetuses. Lanes 5, 6 and 8, ewes with male fetuses. female fetuses were correctly identified by AMEL gene sex determination. The number of male fetuses identified by this gene was 19 out of 20 (95%). The ovine AMEL gene PCR for sex determination had a sensitivity and specificity of 97.82% in this study. Out of 45 samples used in this study, 8 samples were twin pregnancies. One ewe carrying two female fetuses showed a PCR pattern like single female fetus and the ewe bearing twins with both sexes indicated a male-like PCR pattern. Excluding the twin pregnancies, the efficiency of 12 A. Saberivand, S. Ahsan / Animal Reproduction Science 164 (2016) 9–13 Table 1 Ovine fetal sex determination by PCR using both SRY and AMEL gene primers from free fetal DNA of maternal blood plasma in 45 pregnant ewes. Pregnancy trimester Phenotypic sex PCR detected sex by SRY gene primers PCR detected sex by AMEL gene primers Specimen no. 1 3 1 1 2 2 3 1 2 1 3 1 2 3 1 2 3 1 1 1 3 3 3 2 1 1 3 2 2 1 2 1 2 3 2 3 2 3 2 3 2 3 3 M F M M F F M F M M M M F F M F F M F M F F M M F F F F M F F M M M F F F F M F F M M M F M M M F M F M M M F F F M F F M F M F F M M F M F F M F F M M M F F F F M F F M M M F M M F F M F M M M M F F M F F M F M F F M M M F F F M F F M M F F F F F M F F M M 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 using both genes in a PCR reaction to determine fetal sex on the maternal plasma was 95.48%. 4. Discussion This is the first report of ovine fetal sex determination using both SRY and AMEL genes in maternal circulating cell free DNA in Ghezel breed. The PCR reaction using SRY primers only produced a single band of 171 bp in male pregnancies. While, AMEL primers amplified two bands of 182 and 242 bp in male and a single band of 242 bp in female pregnancies. Maternal circulating cell free DNA was previously used for fetal sex determination and to detect fetal diseases and abnormalities in mammals (Bianchi and Lo, 2001; Guang et al., 2009; Davoudi et al., 2011; Kadivar et al., 2013). Kadivar et al. (2013) identified fetal sex with the sensitivity and specificity of 100% with no false negative or false positive results using SRY gene in plasma samples of pregnant ewes. Similarly, ovine fetal sex has correctly been identified in 579 out of 580 samples using SRY gene (Dervishi et al., 2011). In another research (Vanpé et al. (2013) both SRY and AMEL genes were used to successfully identify fetal sex in 3 lemur species. Reliable ovine fetal sex determination could be a key element in ovine management and breeding programs. The traditional methods of fetal sex determination are uncertain and time-consuming and most of them are invasive and have potential risk of pregnancy damage. PCR using the sequence differences between Y and X chromosomes in male and female sex provides precise fetal sex determination. Successful isolation and quantification of cell free fetal DNA from maternal plasma is dependent on plasma harvesting from whole blood samples, DNA extraction from harvested plasma; and PCR quantification or detection of a paternally inherited sequence (Maron et al., 2007). Often the quantity of samples such as hair, urine, skin or fetal cells in maternal circulation for DNA extraction is very low. Therefore, it is important, especially in forensics and crime investigations, to use methods and tools that are able to extract DNA from such a small amount of samples and, to detect the specific sequences in the small quantity of samples (Vanpé et al., 2013). In wild animals, especially where they are in the risk of extinction, safe fetal sex determination using molecular methods without compromising fetal life is crucial (Waits and Paetkau, 2005). Application of both SRY and AMEL genes in maternal circulation may be useful where female embryo’s genotype is XY and due to lack of SRY gene sequence its sex is incorrectly determined as female. There are some reported evidences in humans that infertile males with XX genotype have determined as female using Y chromosome-specific variants (Rajender et al., 2006). DNA contamination with human origin some time could be a problem in sex determination with SRY-specific variants. Therefore, using AMEL gene can overcome this issue as human AMEL gene primer produces different product size of animal AMEL gene (Pfeiffer and Brenig, 2005). The main drawback of this method is the failure to detect the gender of twins. As Ghezel has a twining rate of 20% (Mohammadi and Saberivand, 2012) and there are many ovine breeds that have very high twining rate, this method is not able to distinguish between female fetus and mother AMEL-X product. More investigations are required to develop methods capable of identifying DNA of female fetus from maternal DNA. Multiplex PCR including both genes primers would have been very accurate method if the annealing temperature of the primers were set to be very similar. Quantitation of a PCR product from a plasma sample of pregnant ewe against a verified DNA sample from the same dam in the quantitative real-time PCR using AMEL gene primers might be useful. Acknowledgments This work was supported by funds granted by the Vice Chancellor for Research of Urmia University. The authors A. Saberivand, S. Ahsan / Animal Reproduction Science 164 (2016) 9–13 thank Dr. A. Ghadami and Dr. P. Aparnak for their collaboration in sampling and data collection. References Aasen, E., Medrano, J.F., 1990. Amplification of the ZFY and ZFX genes for sex identification in humans, cattle, sheep and goats. Biotechnology 8, 1279–1281. Ali, A., 2004. Effect of gestational age and fetal position on the possibility and accuracy of ultrasonographic fetal gender determination in dairy cattle. Reprod. Domest. Anim. 39, 190–194. Appao Rat, K.B.C., Totey, S.M., 1999. Cloning and sequencing of buffalo male-specific repetitive DNA: sexing of in-vitro developed buffalo embryos using multiplex and nested polymerase chain reaction. Theriogenology 51, 785–797. Bianchi, D.W., Lo, Y.M., 2001. Fetomaternal cellular and plasma DNA trafficking: the Yin and the Yang. Ann. N. Y. Acad. Sci. 945, 119–131. Davoudi, A., Seighalani, R., Aleyasin, S.A., Tarang, A., Radjabi, R., Tahmoressi, F., 2011. The application of amplified TSPY and amelogenin genes from maternal plasma as a non-invasive bovine fetal DNA diagnosis. EurAsian J. BioSci. 5, 119–126. da Cruz, A.S., Silva, D.C., Costa, E.O.A., de M-Jr.c.P., da Silva, C.C., Silva, D.M., da Cruz, A.D., 2012. Cattle fetal sex determination by polymerase chain reaction using DNA isolated from maternal plasma. Anim. Reprod. Sci. 131, 49–53. de Freitas Neto, L.M., dos Santos, M.H., de Aguiar Filho, C.R., de Almeida Irmão, J.M., Caldas, E.L., Neves, J.P., et al., 2010. Ultrasonographic fetal sex identification in pregnant sheep derived from natural mating and embryo transfer. J. Reprod. Dev. 56, 347–350. Dervishi, E., Sanchez, P., Alabart, J.L., Cocero, M.J., Folch, J., Calvo, J.H., 2011. A suitable duplex PCR for ovine embryo sex and genotype of PrnP gene determination for MOET-based selection programmes. Reprod. Domest. Anim. 46, 999–1003. Ennis, S., Gallager, T.F., 1994. A PCR-based sex-determination assay in cattle based on the bovine amelogenin locus. Anim. Genet. 25, 425–427. Fajfar-Whetstone, C.J., Lane Rayburn, A., Schook, L.B., Wheeler, M.B., 1993. Sex determination of porcine preimplantation embryos via Y-chromosome specific DNA sequence. Anim. Biotechnol. 4, 183–193. Guang, D.X., Yin, X., Jun, M., Ning, G., Li, G.W., 2009. Early pregnancy diagnosis and early prenatal sex determination by maternal plasma cell-free nucleic acids from placenta or fetus. Jiangsu J. Agric. Sci. 25, 1420–1422. Handyside, A.H., Pattinson, J.K., Penketh, R.J.A., Delhanty, J.D.A., Winston, R.M.L., Tuddenham, E.G.D., 1989. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet 18, 347. Jimenez, D.F., Tarantal, A.F., 2003. Quantitative analysis of male fetal DNA in maternal serum of gravid rhesus monkeys (Macaca mulatta). Pediatr. Res. 53, 18–23. Kadivar, A., Hassanpour, H., Mirshokraei, P., Azari, M., Gholamhosseini, K., Karami, A., 2013. Detection and quantification of cell-free fetal DNA in ovine maternal plasma; use it to predict fetal sex. Theriogenology 79, 995–1000. Kageyama, S., Yoshida, I., Kawakura, K., Chikuni, K., 2004. A novel repeated sequence located on the bovine Y chromosome: its 13 application to rapid and precise embryo sexing by PCR. J. Vet. Med. Sci. 66, 509–514. King, W.A., 1984. Sexing embryos by cytological methods. Theriogenology, 17–21. Kunieda, T., Xian, M., Kobayashi, E., Imamichi, T., Moriwaki, K., Toyoda, Y., 1992. Sexing of mouse preimplantation embryos by detection of Y chromosome-specific sequences using polymerase chain reaction. Biol. Reprod. 46, 692. Lo, Y.M., Tien, M.S.C., Lau, T.K., et al., 1998. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am. J. Hum. Genet. 62, 768–775. Lo, Y.M., Corbetta, N., Chamberlain, P.F., Rai, V., Sargetn, I.L., Redman, C.W., 1997. Presence of fetal DNA in maternal plasma and serum. Lancet 350, 485–487. Mandel, P., Métais, P., 1948. Les acides nucléiques du plasma sanguine chez l’homme. Acad. Sci. Paris 142, 241–243. Mara, L., Pilichi, S., Sanna, A., Accardo, C., Chessa, B., Chessa, F., et al., 2007. Sexing of in vitro produced ovine embryos by duplex PCR. Mol. Reprod Dev. 69, 35–42. Maron, J.L., Johnson, K.L., Bianchi, D.W., 2007. In: Thornhill, A. (Ed.), Methods in Molecular Medicine: Single Cell Diagnostics: Methods and Protocols. Humana Press Inc., Totowa, NJ, pp. 51–63. Mohammadi, G., Saberivand, A., 2012. Modified method to extract DNA from mammalian whole blood for PCR-based methods. Sci. Res. Iran. Vet. J. 8, 93–100. Peura, T., Hyttinen, J.M., Turunen, M., Janne, J., 1991. A reliable sex determination assay for bovine preimplantational embryos using polymerase chain reaction. Theriogenology 35, 547–555. Pfeiffer, I., Brenig, B., 2005. X- and Y-chromosome specific variants of amelogenin gene allow sex determination in sheep (Ovis arie) and European red deer (Cervus elaphus). BMC Genet. 6, 16. Piprek, R.P., 2010. Molecular and cellular machinery of gonadal differentiation in mammals. Int. J. Dev. Biol. 54, 779–786. Pomp, D., Good, B.A., Geisert, R.D., Corbin, C.J., Conley, A.J., 1995. Sex identification in mammals with polymerase chain reaction and its use to examine sex effects on diameter of day-10 or -11 pig embryos. J. Anim. Sci. 73, 1408–1415. Rajender, S., Rajani, V., Gupta, N.J., Chakravarty, B., Singh, L., Thangaraj, K., 2006. SRY-negative 46, XX male with normal genitals, complete masculinization and infertility. Mol. Hum. Reprod. 12, 341–346. Swarup, V., Rajeswari, M.R., 2007. Circulating (cell-free) nucleic acids-A promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 581, 795–799. Vanpé, C., Salmona, J., Pais, I., Kun-Rodrigues, C., Pichon, C., Meyler, S.V., Chikhi, L., 2013. Noninvasive molecular sexing: an evaluation and validation of the SRY-and amelogenin-based method in three new lemur species. Am. J. Phys. Anthropol. 150, 492–503. Waits, L.P., Paetkau, D., 2005. Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. J. Wildl. Manag. 69, 1419–1433. Watchel, S.S., 1984. H-Y antigen in the study of sex determination and control of sex ratio. Theriogenology 21, 797–799. Wooding, F.B.P., 1992. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta 13, 101–113.