XML - Ihre Homepage bei Arcor
Werbung

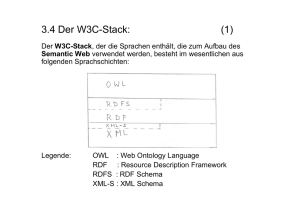
XML Workshop XML – Standardformat für den Austausch von elektronischen Daten in der pharmazeutischen Industrie? Joerg Dillert Senior Consultant March, 30th, 2004 © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. 0. Allgemeines © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. Der Workshop … ist in Germisch! 3 Ein paar Regeln 9.00 – 16.30 Pausen 15,60,15 Handys aus oder Vibration! Toiletten Fluchtwege Fragen - bitte jederzeit 4 1. Einführung Was ist eigentlich XML? Wie ist es entstanden? © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. Handys, Smartphones und PDAs mit integrierter SyncML-Unterstützung Modell Anbieter Gerätetyp Verfügbarkeit Alcatel: ot715 Motorola: A830, A835, V600, E390 Nokia 7250, 6800, 3650, 6220, 9210i, 7650 Samsung SGH-D700 Siemens: S55, SL55, M55, SX1 Sony Ericsson: T68i, T610, P800, Z1010, PEG-NZ90 PDAs: Sony PEG-NX70V, PEG-T675C,PEG-T625C 6 Wir leben im Zeitalter der Buzzwords B2B, B2M, E2B DIA, EMEA, FDA XML, DTD, XSL, SVG Die (Computer) Industrie gibt uns viele neue Wörter jede Woche Schauen Sie mal an Ihren Arbeitsplatz – welches sind denn so Ihre Buzzwords? (SOPs, DCFs, …) 7 ... kennen Sie diese Deutsche Musikgruppe? Smudo MFG 8 Urkundlich erwähnt … SGML Standard Generalized Markup Language ISO 88791 seit 1986 9 The SGML family of markup languages – more buzzwords!! GML Generalized Markup Language Goldfarb, Mosher and Lorie, IBM, 1969 IBM Document Composition Facility DCF (Script) SGML Standardized Generalized Markup Language Content attributes. ISO-8879 first published in 1986 HTML HyperText Markup Language Functional attributes: hyperlink, frame Based on hyperdocument standard definitions CALS Continuous Acquisition and Life-cycle Support Based on DoD MIL-M-28001B standard definitions XML eXtensible Markup Language (Founding father: Dr. Charles F. Goldfarb, IBM) 10 1986 Entwicklung SGML in den IBM Labs in Almaden Charles Goldfarb ISO Standard Überarbeitung 1990, Ziel war eine universell einsetzbare Auszeichnungssprache für Dokumente 11 A brief history of SGML The Evolution of Markup Languages Plain text Font attributes: Bold, underline, italics, font size Document structure attributes: Heading level, index term Document content attributes: Patient age, dosage unit 12 1990 Am Kernforschungszentrum Cern in Genf begann Entwicklung von HTML erster Entwurf 1993, Geburtstunde des Web 1995 überarbeitet HTML Version 2.0 13 1994 Um Wildwuchs zu verhindern – Gründung des World Wide Web Consortium (W3C) primäre Aufgabe: Weiterentwicklung von HTML 14 1998 W3C erkannte, daß mit HTML die Herausforderungen der Zukunft nicht gemeistert werden können Zwischen Zuviel an Markup (SGML) und dem Zuwenig (HTML) sollte der goldene Mittelweg gefunden werden Abschluß des Findungsprozesses – XML 1998 als offizieller Standard verabschiedet 15 2001 W3C verabschiedet als wichtigste Ergänzung die erste Version von XSL (Extensible Stylesheet Language) stellt Regeln zur Umwandlung von XML Dokumenten und ein Vokabular zum Formatieren dieser Dokumente zur Verfügung 2002 Arbeitsentwurf zu XHTML Version 2.0 , Bruch mit HTML 4.0 und XHMTL 1.0 – keine Rückwärtskompatibilität 16 The big advantage of XML You have flexibility - you can define your own TAGS The Parser need only the DTD / Schemas for checking the correctness of your file Readable for everyone Vendor independent (No vendor can impose their own definitions, standards or undocumented formats) License free ... all three types are in ASCII format! (American Standard Code for Information Interchange ) 17 XML and data interchange This kind of information data interchange is the standard in other industries and is called B2B The Germans favourite spare time object ... • is produced Just in Time 18 What we‘ve learned from other industries ... Supplier System Y Car producer System X B2B Server 19 Request Order Delivery Proposal Order confirmation Delivery confirmation Invoicing B2B Server XML is ... The data interchange and document format for now and in the future (E2B, CDISC, CTD) Is in practical use in many industries • E.g. car production industry EVERY system can communicate with another You need only ONE interface per system 20 2. XML eXtensible Markup Language © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. XML XML - eXtensible Markup Language • It is a subset of SGML • It focusses on content (sometimes also structure) • The XML file contains the DATA • It is restricted by TAGs • Example: <messagetype>ICSR</messagetype> 22 An example as a graphic 23 ... and as XML structure <ANA106> <Screening visit> <Inclusion criteria> <Inc1>YES</Inc1> <Inc2>YES</Inc2> <Inc3>YES</Inc3> <Inc4>YES</Inc4> </Inclusion criteria> <Exclusion criteria> <Excl1>NO</Excl1> <Excl2>NO</Excl2> <Excl3>NO</Excl3 <Excl4>NO</Excl4> </Exclusion criteria> <Demographic/Investigator> <Sex>Male</Sex> <DoB>07/26/1966</DoB> <Smoke>Yes</Smoke> <InvNo>128</InvNo> </Demographics/Investigator> ... --- more page(sections> </Screnning visit> <Visit 1> ... --- more blocks </Visit 1> 24 ... </ANA106> --- more visits einfache XML Struktur <?xml version="1.0" encoding="ISO-8859-1"?> <DVMDTagung> <Workshop> <event > <stadt>Ulm</stadt> <ort>MedSchule</ort> </event> </Workshop> </ DVMDTagung > 25 Attribute <?xml version="1.0" encoding="ISO-8859-1"?> <DVMDTagung> <Workshop name=„XML in der Pharmazeutischen Industrie" Leiter=„Joerg Dillert"> <event datum="31.03.2004"> <stadt>Ulm</stadt> <ort>MedSchule</ort> </event> <event datum=„25.06.2004"> <stadt>Berlin</stadt> <ort>PFOffice</ort> </event> </Workshop> </ DVMDTagung > 26 Attribute <?xml version="1.0" encoding="ISO-8859-1"?> <!– zum Kommentieren --> <DVMDTagung> <Workshop name="XML in der Pharmazeutischen Industrie" Leiter=„Joerg Dillert"> <event datum="31.03.2004"> <stadt>Ulm</stadt> <ort>MedSchule</ort> </event> <event datum="25.06.2004"> see in IE <stadt>Berlin</stadt> <ort>PFOffice</ort> </event> </Workshop> </DVMDTagung > 27 see in XML Notepad Characters Character set • Characters that may be represented in XML document – e.g., ASCII character set • Letters of English alphabet • Digits (0-9) • Punctuation characters, such as !, - and ? 28 Character Set XML documents may contain • Carriage returns • Line feeds • Unicode characters – Enables computers to process characters for several languages 29 Characters vs. Markup XML must differentiate between • Markup text – Enclosed in angle brackets (< and >) • e.g,. Child elements • Character data – Text between start tag and end tag • e.g., Fig. 5.1, line 7: Welcome to XML! 30 White Space, Entity References and Built-in Entities Whitespace characters • Spaces, tabs, line feeds and carriage returns – Significant (preserved by application) – Insignificant (not preserved by application) • Normalization - Whitespace collapsed into single whitespace character - Sometimes whitespace removed entirely <markup>This is character data</markup> after normalization, becomes <markup>This is character data</markup> 31 White Space, Entity References and Built-in Entities (cont.) XML-reserved characters • Ampersand (&) • Left-angle bracket (<) • Right-angle bracket (>) • Apostrophe (’) • Double quote (”) Entity references • Allow to use XML-reserved characters – Begin with ampersand (&) and end with semicolon (;) • Prevents from misinterpreting character data as markup 32 White Space, Entity References and Built-in Entities (cont.) Build-in entities • Ampersand (&amp;) • Left-angle bracket (&lt;) • Right-angle bracket (&gt;) • Apostrophe (&apos;) • Quotation mark (&quot;) • Mark up characters “<>&” in element message <message>&lt;&gt;&amp;</message> see in IE 33 Using Unicode in an XML Document XML Unicode support • e.g., displays Arabic words – Arabic characters • represented by entity references for Unicode characters 34 <?xml version = "1.0"?> <welcome> <from> &#1583;&#1575;&#1610;&#1578; &#1614;&#1604;&#1571;&#1606;&#1583; </from> XML document that contains Arabic words <subject> &#1571;&#1607;&#1604;&#1575;&#1611; &#1576;&#1603;&#1605; &#1601;&#1610;&#1616; &#1593;&#1575;&#1604;&#1605; </subject> </welcome> see in IE 35 Markup XML element markup • Consists of – Start tag – Content – End tag • All elements must have corresponding end tag <img src = “img.gif”> is correct in HTML, but not XML • XML requires end tag or forward slash (/) for termination <img src = “img.gif”></img> or <img src = “img.gif”/> is correct XML syntax 36 Markup (cont.) Elements • Define structure • May (or may not) contain content – Child elements, character data, etc. Attributes • Describe elements – Elements may have associated attributes • Placed within element’s start tag • Values are enclosed in quotes – Element car contains attribute doors, which has value “4” <car doors = “4”/> 37 Markup (cont.) Processing instruction (PI) • Passed to application using XML document • Provides application-specific document information • Delimited by <? and ?> 38 1 <?xml version = "1.0"?> 2 3 <!-- Fig. 5.5 : usage.xml 4 --> <!-- Usage of elements and attributes --> 5 6 <?xml:stylesheet type = "text/xsl" href = "usage.xsl"?> 7 8 <book isbn = "999-99999-9-X"> 9 <title>Deitel&amp;s XML Primer</title> 10 11 <author> 12 <firstName>Paul</firstName> 13 <lastName>Deitel</lastName> 14 </author> 15 16 <chapters> 17 <preface num = "1" pages = "2">Welcome</preface> 18 <chapter num = "1" pages = "4">Easy XML</chapter> 19 <chapter num = "2" pages = "2">XML Elements?</chapter> 20 <appendix num = "1" pages = "9">Entities</appendix> 21 </chapters> 22 23 <media type = "CD"/> 24 </book> 39 PI discussed later CDATA Sections CDATA sections • May contain text, reserved characters and whitespace – Reserved characters need not be replaced by entity references • Not processed by XML parser • Commonly used for scripting code (e.g., JavaScript) • Begin with <![CDATA[ • Terminate with ]]> see in IE 40 1 CDATA <?xml version = "1.0"?> 2 3 <!-- Fig. 5.7 : cdata.xml --> 4 <!-- CDATA section containing C++ code --> 5 6 <book title = "C++ How to Program" edition = "3"> 7 8 <sample> 9 // C++ comment 10 if ( this-&gt;getX() &lt; 5 &amp;&amp; value[ 0 ] != 3 ) 11 12 cerr &lt;&lt; this-&gt;displayError(); </sample> 13 14 <sample> 15 <![CDATA[ 16 17 // C++ comment 18 if ( this->getX() < 5 && value[ 0 ] != 3 ) 19 cerr << this->displayError(); 20 ]]> 21 </sample> 22 23 C++ How to Program by Deitel &amp; Deitel 24 </book> 41 CDATA (cont.) 42 XML Namespaces Naming collisions • Two different elements have same name <subject>Math</subject> <subject>Thrombosis</subject> Namespaces • Differentiate elements that have same name <school:subject>Math</school:subject> <medical:subject>Thrombosis</medical:subject> – school and medical are namespace prefixes • Prepended to elements and attribute names • Tied to uniform resource identifier (URI) - 43 Series of characters for differentiating names XML Namespaces (cont.) Creating namespaces • Use xmlns keyword xmlns:text = “urn:deitel:textInfo” xmlns:image = “urn:deitel:imageInfo” – Creates two namespace prefixes text and image – urn:deitel:textInfo is URI for prefix text – urn:deitel:imageInfo is URI for prefix image • Default namespaces – Child elements of this namespace do not need prefix xmlns = “urn:deitel:textInfo” 44 XML namespace - no default 1 <?xml version = "1.0"?> 2 3 <!-- Fig. 5.8 : namespace.xml --> 4 <!-- Namespaces --> 5 6 <directory xmlns:text = "urn:deitel:textInfo" 7 xmlns:image = "urn:deitel:imageInfo"> 8 9 <text:file filename = "book.xml"> 10 <text:description>A book list</text:description> 11 </text:file> 12 13 <image:file filename = "funny.jpg"> 14 <image:description>A funny picture</image:description> 15 <image:size width = "200" height = "100"/> 16 </image:file> 17 18 </directory> 45 XML namespace with default 1 <?xml version = "1.0"?> 2 3 <!-- Fig. 5.9 : defaultnamespace.xml --> 4 <!-- Using Default Namespaces --> 5 6 <directory xmlns = "urn:deitel:textInfo" 7 default xmlns:image = "urn:deitel:imageInfo"> 8 9 10 11 <file filename = "book.xml"> <description>A book list</description> </file> 12 13 <image:file filename = "funny.jpg"> 14 <image:description>A funny picture</image:description> 15 <image:size width = "200" height = "100"/> 16 </image:file> 17 18 </directory> 46 needs full name 3. DTD und Schemas © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. DTD / Schema DTD – Document Type Definition • Sometimes also called the ‘Document Type Description‘ Today you have Schemas • Schemas are more detailed • Comes back to XML Contains the ‘grammar’ of an XML document • The parser (a program) checks the correctness of the XML file based on the DTD / Schemas – numerics – characters ... 48 Parsing / Validieren Correct (well-formed) file XML file DTD Schemas 49 DTDs vs. Schemas beschreiben den prinzipiellen Aufbau von Dokumenten eines bestimmten Typs können entweder mit DTDs (Document Type Definitions) oder XML-Schemata spezifiziert werden DTDs wurden von SGML übernommen und sind Teil von XML 1.0. XMLSchema sind ein eigener W3C-Standard. 50 DTDs vs. Schemas (2) XML-Schemata sind ausdrucksstärker DTDs sind kompakter und lesbarer DTDs für Spezifikation von Text-Dokumenten ausreichend XML-Schemata zur Spezifikation von Daten besser geeignet. 51 4. DTDs © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. Wie könnte die DTD aussehen? <BookStore> <Book> <Title>My Life and Times</Title> <Author>Paul McCartney</Author> <Date>July, 1998</Date> <ISBN>94303-12021-43892</ISBN> <Publisher>McMillin Publishing</Publisher> </Book> </BookStore> Ein BookStore soll mindestens ein Buch enthalten. Die ISBN soll optional, alle anderen Kind-Elemente sollen obligatorisch sein. 53 Die DTD für den BookStore <!ELEMENT BookStore (Book+)> <!ELEMENT Book (Title, Author, Date, ISBN?, Publisher)> <!ELEMENT Title (#PCDATA)> <!ELEMENT Author (#PCDATA)> <!ELEMENT Date (#PCDATA)> <!ELEMENT ISBN (#PCDATA)> <!ELEMENT Publisher (#PCDATA)> ähnelt einer regulären Grammatik 54 Deklaration <!ELEMENT BookStore (Book+)> <BookStore> <Book> </Book> <Book> </Book> </BookStore> deklariert das Element BookStore BookStore hat mindestens ein Kind-Element Book. Außer Book darf BookStore keine Kind-Elemente haben. + bezeichnet n Wiederholung des vorstehenden Elementes mit n >= 1. * bezeichnet n Wiederholung mit n >= 0. 55 Deklaration (2) <!ELEMENT Book (Title, Author, Date, ISBN?, Publisher)> <Book> <Title>…</Title> <Author>…</Author> <Date>…</Date> <ISBN>…</ISBN> <Publisher>…</Publisher> deklariert das Element Book </Book> Title, Author, Date, ISBN und Publisher erscheinen (in dieser Reihenfolge) als Kind-Elemente von Book. Außer diesen darf Book keinen anderen Kind-Element haben. , bezeichnet Sequenz (Aufeinanderfolge) von Elementen. ? bedeutet, dass das vorstehende Element in der Sequenz optional ist. 56 Rekursive Darstellung <!ELEMENT BookStore (Book | (Book, BookStore))> rekursive Deklaration von BookStore Bookstore besteht entweder aus genau einem KindElement Book oder hat zwei Kind-Elemente, nämlich Book und BookStore | bezeichnet Auswahl (Disjunktion) Beachte: Diese rekursive Deklaration ist nicht äquivalent zur vorherigen: • <! ELEMENT BookStore (Book+)> 57 Deklaration der Elemente <!ELEMENT Title (#PCDATA)> <!ELEMENT Author (#PCDATA)> <!ELEMENT Date (#PCDATA)> <!ELEMENT ISBN (#PCDATA)> <!ELEMENT Publisher (#PCDATA)> <Title>My Life and Times</Title> <Author>Paul McCartney</Author> <Date>July, 1998</Date> <ISBN>94303-12021-43892</ISBN> <Publisher>McMillin Publishing</Publisher> 58 #PCDATA bezeichnet unstrukturierter Text ohne reservierte Symbole wie „<“. Vordefinierte Datentypen Zur Festlegung von Element-Inhalten stehen drei vordefinierte Datentypen (engl. built-in datatypes) zur Verfügung: • #PCDATA: unstrukturierter Text ohne reservierte Symbole. • EMPTY: Der Element-Inhalt ist leer. Das Element kann allerdings Attribute haben. <!ELEMENT br EMPTY> <br/> • ANY: beliebige XML-Strukturen <!ELEMENT title ANY> Beachte: Gängige Datentypen, wie INTEGER oder FLOAT stehen nicht zur Verfügung. 59 Deklaration von Attributen <!ATTLIST BookStore version CDATA #IMPLIED "1.0"> Das Element BookStore hat ein Attribut version. Außer version hat BookStore keine weiteren Attribute. Das Attribut version ist vom Typ String (CDATA). #IMPLIED: Das Attribut ist optional. "1.0" ist der Standard-Wert. #REQUIRED: Das Attribut ist obligatorisch. #FIXED: Das Attribut hat immer den gleichen Wert. 60 Deklaration von Attributen (2) <!ATTLIST Author gender (male | female) "female"> Das Element Author hat ein Attribut gender und keine weiteren Attribute. Das Attribut hat entweder den Wert male oder female (Aufzählungstyp). "female" ist der Standard-Wert von gender. 61 Datentypen für Attribute Neben Strings (CDATA) und Aufzählungstypen stehen im wesentlichen folgende Datentypen zur Verfügung: NMTOKEN: ein String, der den Namenskonventionen von XML entspricht NMTOKENS: ein Liste von solchen Namen, jeweils getrennt durch ein Leerzeichen ID: Bezeichner, der den Namenskonventionen von XML entspricht und innerhalb des Dokumentes eindeutig ist. IDREF: eine Referenz auf einen eindeutigen Bezeichner IDREFS: eine Liste von solchen Referenzen 62 ID/IDREF <!ATTLIST Author key ID #IMPLIED keyref IDREF #IMPLIED> Author kann ein Attribut key haben. Das Attribut key muss eindeutig sein: Attribute mit dem Typ ID dürfen niemals den gleichen Wert haben. Author kann auch ein Attribut keyref haben. Der Wert von keyref muss eine gültige Referenz darstellen: Der Wert von keyref muss als Wert eines Attributes mit dem Typ ID existieren. 63 ID/IDREF (2) <?xml version="1.0" encoding="UTF-8"?> <!DOCTYPE BookStore SYSTEM "BookstoreWithAttributes.dtd"> <BookStore> <Book> <Title>Text</Title> <Author key="k1">Text</Author> <Date>Text</Date> <Publisher>Text</Publisher> </Book> k1 muss eindeutig sein: weder dieses noch ein anderes Attribut mit dem Typ ID darf k1 als Wert haben. <Book> <Title>Text</Title> <Author keyref="k1"/> <Date>Text</Date> <Publisher>Text</Publisher> </Book> </BookStore> 64 k1 muss existieren: ein Attribut mit dem Typ ID muss den Wert k1 haben. Well-formed and valid Ein XML-Dokument heißt wohlgeformt, wenn es den syntaktischen Regeln des entsprechenden W3CStandards entspricht. Ein XML-Dokument heißt zulässig (engl. valid). bzgl. einer Dokument-Typ-Definition, wenn • 1. das Wurzel-Element des XML-Dokumentes in der DTD deklariert ist und • 2. das Wurzel-Element genau die Struktur hat, wie sie in der DTD festgelegt ist. 65 Nachteil von DTDs Reihenfolge von Kind-Elementen ist festgelegt: <!ELEMENT Book (Title, Author)> dadurch sehr starre Struktur in XML-Dokumenten Um Reihenfolgeunabhängigkeit zu garantieren, müssen alle Permutationen explizit aufgezählt werden: <!ELEMENT Book ((Title, Length) | (Length, Title))> Für n Element gibt es n! verschiedene Permutationen. 66 Noch mehr Nachteile keine XML-Syntax, daher eigene Parser nötig kaum vordefinierte Datentypen, insbesondere für Element-Inhalte keine eigenen Datentypen definierbar keine Namensräume: Bereits existierende DTDs können nur dann kombiniert werden, wenn es keine Namenskonflikte gibt! keine Vererbungshierarchien, nicht objekt-orientiert 67 Elements Summary Overview Tag definitions – <!ELEMENT to - - (#PCDATA)> • PCDATA = Parsed Character Data • Comments – <! - - comment text - - > Tag order – Fixed order • <!ELEMENT head - - (to , from)> INVALID: <head><from>S. Ender</from><to>R. Eceiver</to></head> – Any order • <!ELEMENT head - - (to & from)> VALID: 68 <head><from>S. Ender</from><to>R. Eceiver</to></head> (cont.) Tag occurrence – One occurrence • <!ELEMENT head - - (to)> – One or more occurrences • <!ELEMENT head - - (to+)> – Zero or one occurrence • <!ELEMENT head - - (to?)> – Zero or more occurrences • <!ELEMENT head - - (to*)> 69 5. XML Schemas © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. XML Schemas Ähnlich wie eine DTD legt ein XML-Schema (engl. XML schema) den prinzipiellen Aufbau von XMLDokumenten eines bestimmten Typs fest. DTDs wurden zur Beschreibung von strukturierten (für Menschen lesbare) Text-Dokumenten entwickelt. Für die Beschreibung von Dokumenten/Daten zum Austausch zwischen Computern sind sie allerdings zu ausdrucksschwach. Deshalb wurden XML-Schemata entwickelt. 71 Vorteile Ähnlich wie in vielen Programmiersprachen, steht eine Vielzahl von vordefinierten Datentypen zur Verfügung. Es können auch eigene Datentypen definiert werden. keine eigene Syntax, sondern XML-Schema sind selbst XML-Dokumente objekt-orientiert, erlauben Vererbungshierarchien verwenden Namensräume Reihenfolgeunabhängige Strukturen können einfach definiert werden. 72 Datentypen <location> <latitude>32.904237</latitude> <longitude>73.620290</longitude> <uncertainty units="meters">2</uncertainty> </location> DTD Eine Ortsangabe besteht aus dem Breitengrad, dem Längengrad und einem Maß für die Unsicherheit der beiden Angaben. Ein Breitengrad ist eine Dezimalzahl zwischen -90 und +90. Ein Längengrad ist eine Dezimalzahl zwischen -180 und +180. Das Maß für die Unsicherheit ist eine nicht-negative Zahl. Die Unsicherheit wird entweder in Meter oder in Fuß angeben. Schema 73 Unser DTD als Schema <?xml version="1.0"?> <xsd:schema xmlns:xsd="http://www.w3.org/2001/XMLSchema" targetNamespace="http://www.books.org" xmlns="http://www.books.org" elementFormDefault="qualified"> <xsd:element name="BookStore"> <xsd:complexType> <xsd:sequence> <xsd:element name="Book" maxOccurs="unbounded"> <xsd:complexType> <xsd:sequence> <xsd:element name="Title" type="xsd:string"/> <xsd:element name="Author" type="xsd:string"/> <xsd:element name="Date" type="xsd:string"/> <xsd:element name="ISBN" type="xsd:string"/> <xsd:element name="Publisher" type="xsd:string"/> </xsd:sequence> </xsd:complexType> </xsd:element> </xsd:sequence> </xsd:complexType> </xsd:element> </xsd:schema> 74 Einige Elementtypen Datentyp Beispiel string einfacher Text boolean true,false,1,0 decimal 1,23 float -1E4 32 bit double -1E4 64 bit duration P1Y2M3DT10H30M 1 Jahr 2Monate 3 Tage dateTime 2004-03-22T13:22:22 in date und time separierbar hexBinary 0FB7 (0-9A-FA-F) base64Binary xFhbhg/bsEg= Base64 kodierte Dateien anyURI http://www.phaseforward.com QName x:element1 anyURI:NCName ID, IDREF NCName nur als datentyp von Attributen 75 Erläuterung 12345,66678 DTD <-> Schema Jede DTD kann in ein äquivalentes XML-Schema übersetzt werden. Tools wie XML Spy bieten eine entsprechende Funktionalität an. Umgekehrt gibt es allerdings XML-Schemata, für die es keine äquivalente DTD gibt. XML-Schemata sind also ausdrucksmächtiger als DTDs 76 XML Schema sind selbst XML Dokumente <?xml version="1.0"?> <xsd:schema xmlns:xsd="http://www.w3.org/2001/XMLSchema" targetNamespace="http://www.books.org" xmlns="http://www.books.org" elementFormDefault="qualified"> …. </xsd:schema> Ein XML-Schema ist ein XML-Dokument. Wurzel-Element eines Schemas ist immer „schema“ aus dem W3C-Namensraum „XMLSchema“. Letzteres wird auch Schema der Schemata genannt. 77 ELEMENT <!ELEMENT BookStore BookStore (Book+)> <xsd:element name="BookStore"> <xsd:complexType> <xsd:sequence> <xsd:element name="Book" type="BookType„ minOccurs="1„ maxOccurs="unbounded"/> </xsd:sequence> </xsd:complexType> </xsd:element> Standard-Werte für minOccurs und maxOccurs jeweils 1 minOccurs="1" kann also weggelassen werden! 78 <!ELEMENT Book (Title, Author+, Date, ISBN?, Publisher)> <xsd:complexType name="BookType"> <xsd:sequence> <xsd:element name="Title" type="xsd:string"/> <xsd:element name="Author" type="xsd:string" maxOccurs="unbounded" /> <xsd:element name="Date" type="xsd:string"/> <xsd:element name="ISBN" type="xsd:string" minOccurs="0" /> <xsd:element name="Publisher" type="xsd:string"/> </xsd:sequence> </xsd:complexType> 79 Komplettes Schema <?xml version="1.0"?> <xsd:schema xmlns:xsd="http://www.w3.org/2001/XMLSchema" targetNamespace="http://www.books.org" xmlns="http://www.books.org" elementFormDefault="qualified"> <xsd:element name="BookStore"> <xsd:complexType> <xsd:sequence> <xsd:element name="Book" maxOccurs="unbounded"> <xsd:complexType> <xsd:sequence> <xsd:element name="Title" type="xsd:string"/> <xsd:element name="Author" type="xsd:string"/> <xsd:element name="Date" type="xsd:string"/> <xsd:element name="ISBN" type="xsd:string"/> <xsd:element name="Publisher" type="xsd:string"/> </xsd:sequence> </xsd:complexType> </xsd:element> </xsd:sequence> </xsd:complexType> </xsd:element> </xsd:schema> 80 Software zum Validieren xerces by Apache (API) • http://www.apache.org/xerces-j/index.html MSXML (API) • http://www.microsoft.com XML Spy (GUI) • http://www.xmlspy.com http://www.w3.org/XML/Schema#Tools 81 6. XSL(T) eXtensible Stylesheet Language © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. XSL Regeln zum Umwandeln von Dokumenten nicht nur nach HTML 83 Transformation Correct (well-formed) file XML file XSL(T) PDF DTD Schemas (X)HTML SVG XML XLS TXT 84 ..… XSL workshop Click here – Tour daten Cocoon 85 7. SVG Scalable Vector Graphics © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. SVG sind Graphiken, die auf XML beruhen spezielle Anzeigeprogramme notwendig (Browser Plugin, Adobe SVG Viewer) 87 8. Path, XQuery © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. XPath XPath (XML Path Language) ist eine Abfrage-Sprache, um Teile eines XMLDokumentes zu adressieren. XPath 1.0 bildet die Grundlage der in XSLT verwendeten Patterns sowie von XPointer. Die nächste Version, XPath 2.0, wird zudem die Grundlage der XML-Abfragesprache XQL / XQuery / XML Query bilden. Anatomie von XPath XPath betrachtet, ähnlich dem DOM, XML-Dokumente als Baumstruktur mit Knoten. XPath unterscheidet dabei folgende Knotentypen: • • • • • • • 89 Dokumentwurzel Elemente Attribute Text Namespaces Processing Instructions Kommentare Die Syntax ist angelehnt an die der UNIX-Pfadangaben. XPath Beispiel Beispiel <dok> <!-- ein XML-Dokument --> <kap title="Nettes Dokument"> <pa>Ein Absatz</pa> <pa>Noch ein Absatz</pa> <pa>Und noch Absatz</pa> <pa>Nett, oder?</pa> </kap> <kap title="Zweites Kapitel"> <pa>Ein Absatz</pa> </kap> </dok> Beispiele für XPath-Ausdrücke: //pa selektiert alle pa-Elemente auf allen Ebenen kap[@title="Nettes Dokument"]/pa selektiert alle Absätze des Kapitels "Nettes Dokument". 90 siehe CD XPath dokumentation 91 9. Technische Anwendungen Webservices, Data Interchange, etc © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. XML ist der Standard für alles Webservices Programmierumgebungen Data Interchange Systeme 93 Webservices WebStandard • a standardized interface, API based on XML • independent, readable several security issues fixed in the meantime new thinking of ‚firewalling‘ • it‘s now content oriented 94 Programmierumgebung .NET • Visual Studio .NET Java • Eclipse N(Ant) Build tool für beide Welten 95 Data Interchange B2B E2B CDISC eCTD 96 Systeme MathML ChemML Phase Forwards MedML Open Office 97 MathML 98 Bsp: SyncML 99 Handys, Smartphones und PDAs mit integrierter SyncML-Unterstützung Modell Anbieter Gerätetyp Verfügbarkeit Alcatel: ot715 Motoroal: A830, A835, V600, E390 Nokia 7250, 6800, 3650, 6220, 9210i, 7650 Samsung SGH-D700 Siemens: S55, SL55, M55, SX1 Sony Ericsson: T68i, T610, P800, Z1010, PEG-NZ90 PDAs: Sony PEG-NX70V, PEG-T675C,PEG-T625C 100 10. E2B © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. Welcome to the Electronic World for the Safety of the Circle of Life! 102 10.1. ICH and where the name ‘E2B’ came from © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. E2B – who is working with these standards? Who is currently working with E2B? Who knows the content of E2B? 104 Introduction to ICH and Efficacy Topics ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 105 Introduction to ICH and Efficacy Topics ICH Representation • Countries and Regions directly represented at ICH are the European Union, the USA and Japan. • Canada, other European countries and other countries are represented by observers: • Canada: Drugs Directorate, Health Canada • Other European countries European Free Trade Area (EFTA) , represented at the ICH by Switzerland • Other countries: World Health Organisation (WHO) Additional Observer: International Federation of Pharmaceutical Manufacturers Association 106 Introduction to ICH and Efficacy Topics ICH Structure Three areas (European Union, USA, Japan) - Regulatory Bodies for each area - Research-base Industry for each area USA EU Japan 107 Six Parties: Food & Drug Administration European Commission Ministry of Health, Labor and Welfare, Japan Pharmaceutical Research and Manufacturers of America European Federation of Pharmaceutical Industries and Associations Japanese Pharmaceutical Manufacturers Association Introduction to ICH and Efficacy Topics ICH Process Step 1: Consensus building Step 2: Start of Regulatory Action Step 3: Regulatory Consultation Step 4: Adoption of a Tripartite Harmonised Text Step 5: Implementation 108 The ICH website 109 Where the name comes from ... Structure of the ICH documents: Q – Quality topics “Those relating to chemical and pharmaceutical Quality Assurance“ S – Safety topics “Those relating to in vitro and in vivo pre-clinical studies“ E – Efficacy topics “Those relating to clinical studies in human subject“, M – Multidisciplinary Topics “Topics covering more than one area“ 110 Where the name comes from ... (cont.) all EFFICACY documents E1 – Exposure E2 – Clinical Safety E3 – Study reports E4 – Dose response E5 – Ethnic factors E6 – GCP E7 – Special population E8, 9, 10 – Clinical Trial Design E11 – Pediatrics 111 E12 – Therapeutics Categories Where the name comes from ... (cont.) all CLINICAL SAFETY documents E2A – Definitions and Standards for Expedited Reporting E2B – Data Elements for Transmission of ADR reports E2B(M) – Data Elements for Transmission of Individual Case Safety Reports • Maintenance, last release February 2001 E2C – Periodic Safety Update Reports 112 Where the name comes from ... (cont.) Multidisciplinary Topics M1 – Medical Terminology (MedDRA) M2 – Electronic Standards for Transmission of Regulatory Information (ESTRI) M3 – Timing of Pre-clinical Studies in Relation to Clinical Trials M4 – The Common Technical Document 113 E2B – E2B(M) E2B contains the definition of all required data elements The first definition was found to be successful and only minor revisions have been made • (language problems, MedDRA versioning) Pilot studies demonstrated succesful usage M2 is another part of the ICH documents ... 114 M2 Electronic transmission of ICSR Message Specification M2 is part of ‘Multi-disciplinary Topics’ in the ICH structure It is the technical description of E2B(M) and defines the interchange format It exists in three different versions • 1.0 • 2.0 • 2.1 -> last release Nov. 2000, final version Feb. 2001 From the technical point of view: ICH ICSR DTD Version 2.1 115 Interchange with E2B ... is an accepted international standard for interchange the ICSR (Individual Case Safety Report) data between the interested groups Interchange between • from authority to authority • pharmaceutical industry to authorities • inside a company • B2B between companies (ex. pharmaceutical with CRO‘s) 116 10.2. The content of an ICSR document © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. The ICH-E2B/M2 Message Structure M ICH ICSR Message Header A Information on the Safety Report A.1 Identification of the Case Safety Report A.2 Primary Source of Information A.3.1 Sender A.3.2 Receiver B 118 Information on the Case B.1 Patient Characteristics B.2 Reaction(s) / Event(s) B.3 Results of Tests and Procedures B.4 Drug(s) Information B.5 Narrative Case Summary and Other Information E2B – example chapter A.1 IDENTIFICATION OF CASE RECORDS Total of 14 different fields Examples: Unique Sender-ID, country information (primary source & where the case came up), date, report type, seriousness, linked with another case 119 E2B – example field A.1.4 TYPE OF REPORT Spontaneous report Report from study Other (... unclear from the literature report ...) Not available to sender (unknown) 120 Repeating records In every Safety Report some repeating items are possible: • example: – Events (B.2) – Drugs (B.4) – Primary source (A.2) 121 safetyreport area (partial) 122 patient area (partial) 123 reaction area 124 drug area (partial) 125 10.3. E2B(M) and M2 – description of the model © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. The technical description Is found in the M2 document Description of the: • Safety report • Acknowlegdement message Contains a description: How to write an ICSR 127 problem? 128 language attribute and the DCL problem 5 characteristics fonts Defined in an DCL file • Declaration language file, which is SGML based MLHW – two files for E2B submission 129 MedDRA Is a basic requirement for E2B Which version? How is it implemented in an E2B file? LLT or PT? at the Notes for Guidance Reg. Elec. Transmission of ICSR in Pharmacovigilance 130 Problem: Unitlists (codelists) Defined in the E2B(M) document • Mass, Volume, Radioactivity, Other • Interval list • Route of Administration list 131 Problem: More than one transmission of an ICSR Unique Case ID Solution in Appendix of the E2B(M) document 132 The steps which need the most time: Map your data ! (Data, Codelists) 133 Clintrace E2b Data Mapping Principles Mapping philosophy • Mapping provides increased flexibility (particularly in view of the upcoming E2b changes) • Mapping allows for addressing field length issues 134 – Note:The standard schema of your drug safety system includes many more data items than the E2b definition Clintrace E2b Data Mapping Principles (cont.) Dependent on the type of item several mapping methods can be distinguished • Direct • • • • 135 Data directly transferable. This can be: Numerical (numbers; dates) Textual (any form of alpha-numerical data) Numeric Data transferable after numeric conversion Example: Weight quantity + unit Weight (in KG) Text Data transferable after text conversion Example: Truncation or Concatenation Coded Data transferable after a code conversion Example: Sex Fixed Standard codes such as date formats Example: Code 102 for complete dates E2b Mapping – Example: Case ID format An automatic Case ID Generation algorithm can be implemented in order to create Case IDs directly compliant with E2b recommendations. Alternatively the mapping can be used to transmit the compliant Case ID. Case ID format for E2b is: country code (2 digits)-company or regulatory namereport number 136 E2B Mapping: Codelists Example: Reporter’s Qualification 1=Physician 2=Pharmacist 3=Other Health Professional 4=Lawyer 5=Consumer or Other Non Health Professional Reporter's Type Code HP SI CN UNK Value Health Professional Study Investigator Consumer Unknown Label Health Professional Study Investigator Consumer Unknown Reporter's Occupation Code PHARMACIST LAWYER Value Pharmacist Lawyer Label Pharmacist Lawyer Allowed Combinations HP/nn HP/empty SI/nn SI/empty HP/PHARMACIST HP/OTHER_HP CN/LAWYER CN/OTHER_NON_HP CN/empty Map to E2B code: 1 (Physician) 1 (Physician) 1 (Physician) 1 (Physician) 2 (Pharmacist) 3 (Other Health Professional) 4 (Lawyer) 5 (Consumer or Other Non HP) 5 (Consumer or Other Non HP) Implementation proposal 137 After sending an ICSR you ... will receive an ACKNOWLEDGMENT Message: • 1. The acknowledgement of receipt • 2. The verification of correctness of the ICSR Also sent out as XML (SGML) 138 The acknowledgement message Split into • ICHICSR MESSAGE HEADER • ACKNOWLEDGMENT – Message acknowledgment (once) – Report acknowledgment (once, many, none) 139 Requirements for your Tracking System you must be able to react to error conditions quickly • transport/communication error – server down – broken line should support more than the standard messages • should include also messages for non-transmission, other possible errors, etc. 140 What does an ICSR file look like? 141 XML in IE XML Notepad The model 10.4. The Electronic Journey © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. The Electronic Journey of your ICSR <ichicsr lang="en"> <safetyreport> <safetyreportid>KAL96.00180</safetyreportid> <primarysourcecountry>US</primarysourcecountry> <transmissiondateformat>102</transmissiondateformat> <transmissiondate>20011119</transmissiondate> <reporttype>2</reporttype> <serious>1</serious> <seriousnessdeath>2</seriousnessdeath> Sender DB Application <seriousnesslifethreatening>2</seriousnesslifethreatening> <seriousnesshospitalization>1</seriousnesshospitalization> <seriousnessdisabling>2</seriousnessdisabling> <seriousnessother>2</seriousnessother> <receivedateformat>102</receivedateformat> <receivedate>19960318</receivedate> <receiptdateformat>102</receiptdateformat> <companynumb>KAL96.00180</companynumb> <primarysource> ICSRACK Gateway <reportertitle>Dr.</reportertitle> <reportergivename>Glenn</reportergivename> Message Ack <reportermiddlename>H.</reportermiddlename> <reporterfamilyname>Wilson</reporterfamilyname> <reportercity>Mossville</reportercity> <reporterstate>TX</reporterstate> <reporterpostcode>71100</reporterpostcode> <reportercountry>US</reportercountry> ..... Software </ichicsr> Receiver DB Application Software 143 10.5. Links © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. Useful links http://www.ich.org http://www.ifpma.org/ http://www.fda.gov/cder/ http://www.eudravigilance.org/ http:// www.fda.gov/cder/m2/ http:// www.bfarm.de http:// www.meddramsso.com 145 11. CDISC © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. Clinical Data Interchange Standards Consortium CDISC is an open, multidisciplinary, non-profit organization committed to the development of worldwide industry standards to support the electronic acquisition, exchange, submission and archiving of clinical trials data and metadata for medical and biopharmaceutical product development. The CDISC mission is to lead the development of global, vendorneutral, platform-independent standards to improve data quality and accelerate product development in our industry. 147 The Current State of Data Transfer Medical Records CRO CRF C Operational Data Submission Pharma Operational Data Submission Data Lab 148 Current State: Costly and Time-consuming Pharma 1 Pharma 2 .. . Pharma N Investigator 1 Investigator 2 .. . Investigator K Lab 1 CRO 1 CRO .. 2 . CRO M 149 Lab .. 2 . Lab L Regulatory Standards to Enable Seamless Data Flow ….from Patient to Reviewers ECG eSubmission for Regulatory Review LAB ODM LAB EDC CRO 150 Operational Database SDS ADaM Benefits of Standardization in our Industry Increase efficiencies of performing clinical trials Improve link between healthcare delivery and clinical research/trials Facilitate ‘business’ processes among investigators, biopharmaceutical companies, CROs, technology vendors, clinical laboratories Provide means for long-term archive of electronic clinical trial data and digital signatures Facilitate reviews of regulatory submissions 151 CDISC models Data Sources • Site CRFs •Laboratories •Contract ODM Research Organizations •Development Partners •Discovery Data Pharma Industry 152 Operational Database •Study Data •Audit Trail •Metadata Submission Data SDM •CRT Datasets •Analysis datasets •Metadata Regulatory Archival Database ODM = Operational Data Model SDM = Submissions Data Model CDISC Sponsors and Members join on a corporate basis (>120 Companies, including all of the top 20 global pharmaceutical companies) 54 members of the Industry Advisory Board, one from each Corporate Sponsor 9 Board members (from AstraZeneca, Aventis, Lilly, Merck, First Consulting Group, SAS, Sanofi-Synthelabo, PAREXEL Europe, and an HL7 liaison) Active groups (~ 40 companies each) in Europe and Japan; group initiated in India 3.75 FTE Operations staff 4 Team facilitators (~3 days/month) 153 Just one section: ODM <xs:element name="ODM"> <xs:complexType> <xs:sequence> <xs:element ref="Study" minOccurs="0" maxOccurs="unbounded"/> <xs:element ref="AdminData" minOccurs="0" maxOccurs="unbounded"/> <xs:element ref="ReferenceData" minOccurs="0" maxOccurs="unbounded"/> <xs:element ref="ClinicalData" minOccurs="0" maxOccurs="unbounded"/> <xs:element ref="Association" minOccurs="0" maxOccurs="unbounded"/> <xs:element ref="ds:Signature" minOccurs="0" maxOccurs="unbounded"/> <xs:any namespace="##other" minOccurs="0" maxOccurs="unbounded"/> </xs:sequence> <xs:attribute name="Description" type="text"/> <xs:attribute name="FileType" type="FileType" use="required"/> <xs:attribute name="Granularity" type="Granularity"/> <xs:attribute name="Archival" type="YesOnly"/> <xs:attribute name="FileOID" type="oid" use="required"/> <xs:attribute name="CreationDateTime" type="datetime" use="required"/> <xs:attribute name="PriorFileOID" type="oidref"/> <xs:attribute name="AsOfDateTime" type="datetime"/> <xs:attribute name="ODMVersion" type="text"/> <xs:attribute name="Originator" type="text"/> <xs:attribute name="SourceSystem" type="text"/> <xs:attribute name="SourceSystemVersion" type="text"/> <xs:attribute name="Id" type="xs:ID"/> <xs:anyAttribute namespace="##other"/> </xs:complexType> <xs:unique name="UC-O-1"> <xs:selector xpath="Study"/> <xs:field xpath="@OID"/> </xs:unique> 154 </xs:element> Major sections of the model 155 Study 156 Metadata are described in the structre itself! 157 Clinical data 158 Subject data use XMP Spy 159 Links www.cdisc.org 160 12. HL7 © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. 13. Ist es Zukunft oder heute schon möglich? © 2003 Phase Forward Incorporated. All rights reserved. Proprietary and Confidential. If you have a serious adverse event during a study ... CRO / Investigator Sponsor ClinTrial Study coordinator runs a study (EDC or paper) SAE CIS B2B server Documentation eMail, SMS ClinTrace yes, SAE Authority (FDA, EMEA) 163 Acknowlegdement Drug safety The (possible) use of XML extracts CRO / Investigator Sponsor Study coordinator CDISC MedML CIS ClinTrial B2B server Documentation WML ClinTrace MedML Authority (FDA, EMEA) 164 E2B Drug safety Links www.w3c.org www.xml.com xml.apache.org … eine Suche bei Google mit den drei Buchstaben ergab ca. 36.400.000 Treffer (und das in Sekunden) …. 165 Questions and Answers 166 THANK YOU! Joerg Dillert Senior Consultant Doebelner Str. 4 12627 Berlin Tel.: +49 - (0) 30 99 28 29 11 Fax: +49 - (0) 30 99 28 29 12 mobile: +49 – (0) 170 448 04 45 [email protected] 167 Nothing beats experience.