VL 3 - Innate PRR and paghocytosis
Werbung
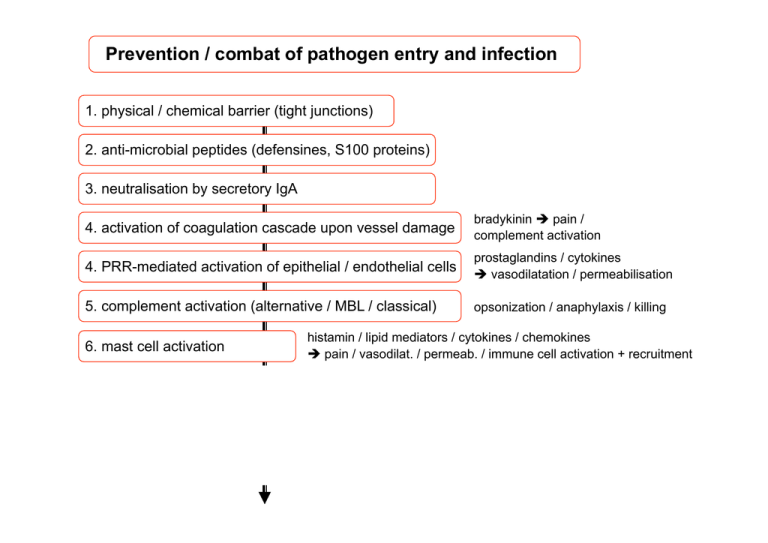
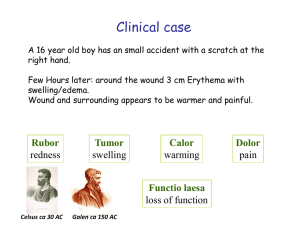
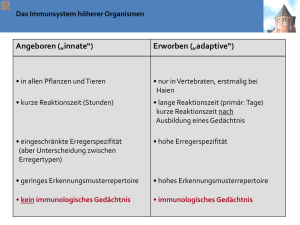
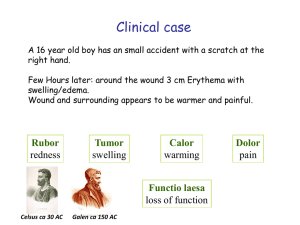
Prevention / combat of pathogen entry and infection 1. physical / chemical barrier (tight junctions) 2. anti-microbial peptides (defensines, S100 proteins) 3. neutralisation by secretory IgA 4. activation of coagulation cascade upon vessel damage bradykinin è pain / complement activation 4. PRR-mediated activation of epithelial / endothelial cells prostaglandins / cytokines è vasodilatation / permeabilisation 5. complement activation (alternative / MBL / classical) opsonization / anaphylaxis / killing 6. mast cell activation histamin / lipid mediators / cytokines / chemokines è pain / vasodilat. / permeab. / immune cell activation + recruitment 7. local PRR-macrophage activation 8. recruitment of neutrophils and more macrophages 9. phagocytosis and killing of pathogens Neutrophils only accumulate in tissues upon injury Homeostasis Staphylococcus infection Prevention / combat of pathogen entry and infection 1. physical / chemical barrier (tight junctions) 2. anti-microbial peptides (defensines, S100 proteins) 3. neutralisation by secretory IgA 4. activation of coagulation cascade upon vessel damage bradykinin è pain / complement activation 4. PRR-mediated activation of epithelial / endothelial cells prostaglandins / cytokines è vasodilatation / permeabilisation 5. complement activation (alternative / MBL / classical) opsonization / anaphylaxis / killing 6. mast cell activation histamin / lipid mediators / cytokines / chemokines è pain / vasodilat. / permeab. / immune cell activation + recruitment 7. local PRR-macrophage activation 8. recruitment of neutrophils and more macrophages 9. phagocytosis and killing of pathogens What kind of PRRs do we know? pathogen Typical Biochemical structures= patterns Injury, mechanical stress, Trauma PAMPs= DAMPs=Alarmine Pathogen Associated molecular patterns Damage associated molecular patterns Pattern recognition receptors = PRRs (soluble and cell-associated) pathogen 3) Sekretion of pro-inflammatory mediators by immune cells (Makrophages, Dendritic cells) bioactive lipids (Thromboxanes, Prostaglandines, leukotriens) Vasodilation Higher blood circulation rubor=redness cytokines Activation of local endothelial cells chemokines attract „gorging“ phagocytic cells tumor=Swelling Synthesis of bioactive Lipids Pharmacological Targets: Phospholipase A2 Cyclooxygenase (COX) Ibuprofen Receptores for PAMPs = Pattern recognition receptors (PRRs)? LPS LBP CD14 ?? NF-κB Pro-inflammatorische Zytokine (z.B. TNF-α) Immune response of the fruit fly (Drosophila) Nüsslein-Volhardt discovery of Toll, Nobel prize 1995 Jules Hoffmann innate immune response in drosophila, Nobel prize 2011 together with Bruce Beutler and Ralph Steinman Drosophila Human/Mice etc • PAMPS-TLR-Specificities • West AP, et al. 2006 in Annu. Rev. CellDev. + Biology TLRs can trigger immune response via 2 different signalling pathways only Myd88 Independent (= TRIF dependent) only Myd88 dependent TLR1/2 TLR2/6 TLR5 TLR7 TLR8 TLR9 TRIF TLR3 TLRs can trigger immune response via 2 different signalling pathways TLRs: Receptors as relays of differences in dangers Output: Patterned responses! TLRs are located in different cell types and compartiments mainly in macrophages and mDCs mainly in pDCs NF-κB TNFα etc. IRF7 IFNβ Nucleus Also other (non-immune) cells (e.g. epithelial cells) can express TLRs Other PRRs – NOD1 and NOD2 as intracellular PRRs How can a virally infected non-immune cell, which does not express TLR3, 7 or 9 sense the viral infection and induce IFNα expression? Alternative (TLR independent) intracellular RNA recognition MAVS signaling pathway. RIG-I or MDA-5 contains 2 N-terminal CARDs and a C-terminal RNA helicase domain that interacts with viral dsRNA (earlyreplication intermediates). Interaction between RIG-I or MDA-5 and MAVS through CARD stimulates nuclear factor (NF)-κB– and interferon (INF) regulatory factor (IRF)– dependent pathways. Toshitaka Yajima, and Kirk U. Knowlton Circulation. 2009;119:2615-2624 Intracellular DNA and RNA sensors Typ-­‐I-­‐Interferone Bildung • Induk@on von IFN-­‐β durch dsRNA (TLR3 oder PKR) in allen Zellen • Verstärkung autokrin oder parakrin über IFN-­‐R , Bildung von IFN-­‐α (wirkt dann unabhängig von NFkB) • Interferon-­‐producing cells (IPC) (1 % der Leukozyten, sehen wie Plasmazellen aus), können 1000x mehr IFN produzieren als andere Zellen) und besitzen verschiedene TLR und differenzieren zu plasmacytoid dendri@c cells (pDCs) Folgen • Bildung von Oligoadenylat-­‐Synthetase (Synthese von 2´-­‐5´-­‐oligoA, ak@viert Endonuklease, baut virale RNA ab) • verstärkt Prolifera@on und Cytotoxizität von NK-­‐Zellen und bewirkt Expression von Liganden für NK-­‐Zellen (MIC-­‐A, MIC-­‐B) • Induk@on von PKR als PRR Inhibition of virus replication by IFNα/β IFNα/β 2-5OA RNAseL Degradation of dsRNA PKR eIF2-p PKR = RNA-dependent protein kinase 2-5OA = 2´-5´ oligoadenylate synthetase translation • Other PRRs – C-­‐type lecDns C-­‐Typ-­‐LekDn-­‐Rezeptoren typisch ist eine Ca2+-­‐abhängige CRD-­‐Domäne (carbohydrate recogni@on domain) • Mφ mannose receptor • DC-­‐SIGN • Dec@n-­‐1 • Mφ mannose receptor is a constantly recycling endocy@c receptor, recogni@on of terminal mannose/ fucose > NAc-­‐Glc (CRD) and sulphated-­‐NAc-­‐Gal • DC-­‐SIGN (DC-­‐specific ICAM3 grabbing non-­‐integrin) is largely restricted to DC, recognises highmannose oligosaccharides • DecDn-­‐1 exhibits specificity for β-­‐(1,3/1,6)-­‐glucans in fungal cell walls expressed on monocytes, MØ, neutrophils and subsets of DC Mφ Mannose Receptor DC-SIGN Dectin-1 http://users.path.ox.ac.uk/~cholt/Lecture1%20handout.pdf Scavenger receptors: PPRs for both infecDous nonself and modified self • • • • Gruppe von Rezeptoren, die modifizierte „low density“ Lipoproteine (mLDL) und anionische Substanzen (z.B. LPS, Lipoteichonsäuren, einige Polynukleo@de) erkennen Aufnahme von acetyliertem und oxidiertem LDL und haben eine Bedeutung bei der Entstehung der Arteriosklerose (CD36 und SR-­‐A) Aufnahme von Listerien, Staphylokokken, Neisserien und apopto@schen Zellen (SR-­‐A) Aufnahme von Streptokokken (MARCO, MAcrophage Receptor with COllagenous structure) one receptor – many ligands LDL receptor ist spezifischer splice-­‐Varianten 2 TM N-­‐term. extrazellulär http://miami.uni-muenster.de/servlets/DerivateServlet/Derivate-131/d_einleitung.pdf Alarmins = DAMPs (damage/danger associated molecular patterns) Necrotic dying cells release danger signals ECM infection MSU Cell death Inflammasome activation ATP Trauma Injury burn DC HMGB1 HSPs inflammation Plasma enzymes HMGB = high mobility group B HSPs = heat shock proteins MSU = monosodium urate; uric acid crystals ECM = extra cellular matrix ECM Degradation (e.g. Hyalunoric acid) TLR Stimulation (also other receptors) cytokines NOD-like receptors – regulators of inflammation NOD1 and NOD2 as intracelluar PAMP sensors NLRPs = inflammasome as DAMP sensor ASC = apoptosis-associated speck-like protein containing a carboxy-terminal CARD Mustererkennungrezeptoren (PRRs) • membrangebundene PRRs scavenger Rezeptoren Toll-­‐like Rezeptoren C-­‐Typ-­‐Lek@n-­‐Rezeptoren Erkennung nach Opsonisierung: mit An@körpern: Fc-­‐Rezeptoren mit C3b: Komplement-­‐Rezeptoren CR3 • cytosolische PRRs NOD-­‐like Rezeptoren (NOD1, NOD2) RIG-­‐I-­‐like RNA-­‐Helikase • sezernierte Proteine Collek@ne: (gehören auch zu C-­‐Typ-­‐Lek@nen) Mannose-­‐bindendes Lec@n (MBL) und surfactant protein A, D (SP-­‐A, SP-­‐D) Ficoline Pentraxine: Serum Amyloid, C-­‐reak@ves Protein, • endozy@erende Rezeptoren • signalgebende Rezeptoren: → adap@ve Immunantwort (Expression von Cytokinen , kos@mulatorischen Proteinen • Komplement-­‐Ak@vierung Summary PRRs Summary PRR signaling Other cytokines (IL-1, TNF-α) stimulate similar pathways IL-17 IL-1R Such cytokines activate (amplify) inflammation – they act as danger signals ! Blutgefäß Gewebe (Haut) Zytokine machen Entzündung Chemokine locken Leukozyten ins entzündete Gewebe Haut (Epithel) Chemokine Infektion Zytokine Gefäßwand (Endothel) transmigration Slow rolling rolling Blutfluss Leukozyt Entzündungsbotenstoffe weisen den Weg zur Infektionsstelle Gewebe (Haut) Haut (Epithel) Infektion PAMPs Komplement aktivierung MΦ DCs Mast zelle Histamin Blutgefäß Zelltod DAMPs PGs Öffnung Vaso Tight dilatation junctions Chemokine Zytokine (z.B. TNFα, IL-1) (z.B. IL-8 = CXCL8) Influx C +AK Induktion Influx Adhäsions Granulozyten moleküle PAMPs = pathogen associated molecular patterns DAMPS = danger/damage associated molecular patterns PRRs = pattern recognition receptors Anlocken und ExtravasaDon von Neutrophilen Glykoprotein-­‐Selek@n CXCL8 (IL-­‐8) wirkt chemotak@sch auf Neutrophile Chemokin-­‐Chemokin-­‐Rezeptor bacterial products (fMLP), C5a, LT-­‐ B4 → Ca2+ → Cytoskelel Integrin-­‐Integrin-­‐Ligand PECAM Neutrophile wandern schneller zum Entzündungort (1 d) als Monozyten (2-­‐3 d) P-­‐selec@n glyco-­‐ protein ligand-­‐1 P(platelet)-­‐SelekDn aus intrazellulären Vesikeln (Weibel-­‐Palade-­‐bodies) Sekunden nach Signal, z. B. Histamin an die Zelloberfläche E(endothelial)-­‐SelekDn: Neusynthese induziert durch TNF-­‐α oder IL-­‐1 über NFκB TNF-­‐α, IL-­‐1 induzieren Bildung von Zell-­‐adhäsions-­‐ molekülen, erhöhte Gefäßpermeabilität (TNF-­‐α) IL-6 IL-12 Ak@vierung von NK-­‐Zellen Leber: Bildung von Akut-­‐ Phase-­‐Proteinen Fieber (IL-­‐1, TNF-­‐α, IL-­‐6) Clinical case development • Afternoon: ague (shivering fever) 38,5 oC. Local inflammation signs enhanced. • Additonally signs of Lymphadenitis and swelling of lymphnodes. • Medic prescribes Antibiotics (Ampicillin) and lokal cooling Kühlung. Start of acute phase response „Warning“ remote response cytokines Tumor Necrosis Factor alpha (TNF-α) Interleukin-1 (IL-1) Pyrogene cytokines Interleukin-6 (IL-6) Granulocytes/Monocytes Colony Stimulating factor GM-CSF Hypothalamus Temperature Set point changes Liver Synthesis of Acute-Phase-Proteins Bone marrow New synthesis of Phagocytic cells Fever u.a. Opsonisation „Linksverschiebung“ Akut Phase Proteine Opsonisierung: Markierung von Erregern durch Bindung an Erregerstrukturen für die erleichterte Phagozytose Beispiele: Collectins: C reaktives Protein (CRP) mannose binding lectin (MBL) serum amyloid protein (SAP) surfactant proteins (SP-A SP-D) Komplement C3 (C3b) Energy for the immune system Adipocyted Lipolyse LipoproteinlipaseActivity Lipid metabolism Muscle cells IL-1 and TNF-alpha Protein metabolism Appetite cachexia and Wasting Makrophage functions 1) Secretion of pro-inflammatory mediators a) cytokine b) chemokines c) bioactive lipids 2. Phase: attraction/accumulation of more/other immune cells 2) Phagocytosis 3) Killing of phagocytosed pathogen Phagocytosis receptors for opsonized pathogenes ? CRP-Rezeptor (CD32??) ? MBL-Rezeptor (CD14??) CRP MBL Fibronektin Komplement (C3b) Integrine „Pathogene Oberfläche“ C3b Antikörper Opsonisierungsprinzip CR1 (CD35) CR3 (CD11b/CD18) CR4 (CD11c/CD18) Fc-Rezeptoren CD16 = FcγRIII CD32 = FcγRII CD64 = FcγRI Makrophagen / Neutrophile Phagozytosis receptors for non-opsonized pathogenes Scavenger-Rezeptoren: SR-A I+II MARCO Lektin-type-Rezeptoren: Mannose Rezeptor β-Glukan-Rezeptor (Dectin-1) LPS-Rezeptor CD14 Phagozytose 1) Aufnahme des Erregers - Pseudopoiden - Aufnahme in die Zelle - Ablösung von Zellmembran 2) Verschmelzung mit Lysosom - Phagolysosom - saurer pH 3) Abtöten des Erregers - oxidative burst (O2-Radikale; NO) 4) Abbau der Erregerbestandteil - Enzyme (Hydrolasen, Proteasen, etc...) Phagozytose 1) Aufnahme des Erregers - Pseudopodien - Aufnahme in die Zelle - Ablösung von Zellmembran 2) Verschmelzung mit Lysosom - Phagolysosom - saurer pH 3) Abtöten des Erregers - oxidative burst (O2-Radikale; NO) 4) Abbau der Erregerbestandteil - Enzyme (Hydrolasen, Proteasen ...) Phagozytose effektiver bei 2 Signalen 1. Fc-Rezeptor 2. Komplementrezeptor + weitere Aktivierung über TLRs / Zytokine / C5a Principles of KILLING ROS-formation ROS = reactive oxygen species „respiratory burst“ Stickstoffoxid-abhängiges Abtöten Examples: Mycobacterium tuberclosis / leprae, Listeria monocytogenes, Salmonella enterica Neutrophil ways of KILLING -­‐ NETosis Mechanism of Neutrophil Extracellular Traps release. Neutrophils are stimulated by contact with bacteria, protozoan, fungi (yeast and hyphae forms) or their products (not shown), leading to: (a) ultrastructural alterations of nuclear shape with chromatin decondensation, swollen and fragmentation of the nuclear membrane, which allow the association of granules and cytoplasmic proteins with the chromatin, and (b) release of extracellular structures consisting of a DNA-backbone, decorated with histones, neutrophil granular and cytoplasmatic proteins, AMPs (NETs), which ensnare and kill microorganisms. Release of neutrophil mediators by degranula@on Mucosal Immunology (2012) 5, 354-366; doi:10.1038/mi.2012.24 Phagozytose nicht möglich-Was nun? Parasiten z.B. Würmer „Frustrierte“ Phagozytose Degranulation C3a C5a Fcε IgE Mastzellen Eosinophile Basophile Phagozytose nicht möglich-Was nun? Hermetisch „Abriegeln“ Granulom Gewebe (Haut) Haut (Epithel) Infektion PAMPs Komplement aktivierung MΦ DCs Mast zelle Histamin Blutgefäß Zelltod DAMPs PGs Öffnung Vaso Tight dilatation junctions Chemokine Zytokine (z.B. TNFα, IL-1) (z.B. IL-8 = CXCL8) Influx C +AK Induktion Influx Adhäsions Granulozyten moleküle PAMPs = pathogen associated molecular patterns DAMPS = danger/damage associated molecular patterns PRRs = pattern recognition receptors Von der angeborenen zur erworbenen Immunität: Transport von Erregerbestandteilen zum Lymphknoten TLR Von der angeborenen zur erworbenen Immunität: Lymphknoten – Treffpunkt der Immunzellen tion infec