zur PDF-Version - CME
Werbung

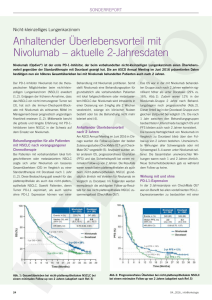
NSCLC (TEIL 1) IMMUNONKOLOGIE (I-O) MITTELS CHECKPOINTINHIBITION: REVOLUTION DER NSCLC-THERAPIE? Wichtige Mediatoren der Immunabwehr Angeboren Antigen unabhängig Antigenpräsentierende Zellen DC Adaptiv Antigen abhängig Lymphokine TLR* PRR* NKG2D IL´e IFN´e CK´e ... Adaptiert nach Woelfel et al, 2014 KIR T-Zellen Fc „natürliche Killer-Zellen“ NK B-Zellen Fab T Fab CTLA-4 CD28 TCR IFN-g Perforin Granzym B Wichtige Mediatoren der Immunabwehr Angeboren Antigen unabhängig Antigenpräsentierende Zellen DC Adaptiv Antigen abhängig Lymphokine TLR* PRR* NKG2D IL´e IFN´e CK´e ... Adaptiert nach Woelfel et al, 2014 KIR T-Zellen Fc „natürliche Killer-Zellen“ NK B-Zellen Fab T Fab Anpassungsfähigkeit Spezifität CTLA-4 Gedächtnis CD28 TCR IFN-g Perforin Granzym B Die T-Zell-vermittelte antitumorale Immunantwort 2 1 Präsentation von Tumorantigen gegenüber der T-Zelle Tumor: Freisetzung von Tumorantigenen 3 4 Erkennung von Tumorantigen durch T-Zellen 5 Zerstörung des Tumors durch T-Zellen Andersen et al, J Invest Dermatol 2006, 126: 32; Pardoll DM, Nat Rev Cancer 2012, 11: 252; Mellman et al, Nature 2011, 480: 480; Heemskerk et al, EMBO J 2013, 32: 194; Boudreau et al, Mol Ther 2011, 19: 841; Janeway et al, Immunobiology: The Immune System in Health and Disease. 6th ed, 2004. T-Zell-Aktivierung und Proliferation Immunsystem und Krebs: Der Prozess des Immunoediting Die drei “E” des Immunoediting beschreiben die Prozesse der Tumorkontrolle durch das Immunsystem und wie der Tumor dieser Kontrolle entkommt. Elimination Tumor-Immunüberwachung CD8+ T-Zelle CD4+ T-Zelle Equilibrium Escape Tumor-Ruhezustand (“Survival of the fittest”) Tumor-Wachstum NK Zelle Treg Tumorzellen Normale Zellen Vesely et al, Ann Rev Immunol 2011, 29: 235 Immune Escape-Mechanismen: Tumore nutzen komplexe Mechanismen, dem Immunsystem zu entkommen A. Ineffektive Tumor-AntigenPräsentation (gp100, MART-1, verringerte MHC-Expression) CD8+ TZelle TCR VEGF APC B. Rekrutierung immunsuppressiver Zellen (regulatorische T-Zellen =Tregs, MDSCs, andere) MHC CTLA-4 MDSC Treg PD-L1 Tumorzellen PD-1 PD-L1 PD-1 TGF-β IDO IL-10 TGF-β ARG1 iNOS TGF-β IL-10 CD4+ D. T-Zell-Checkpoints Vesely et al, Ann Rev Immunol 2011, 29: 235 TZelle CD8+ TZelle C. Sekretion von immunsuppressiven Signalen (z.B. PD-L1, TGF-β, IL-10, und indolamine 2,3dioxygenase [IDO]) Die Immunonkologie mittels Checkpoint-Modifikation als neues therapeutisches Behandlungskonzept1 • • Die konventionellen onkologischen Ansätze sind direkt gegen den Tumor gerichtet.2 Bei der Immunonkologie wird die natürliche Fähigkeit des eigenen Immunsystems genutzt, um den Krebs zu bekämpfen.2 Operation Immunonkologie Strahlentherapie Chemo- & zielgerichtete Therapien 1. DeVita and Rosenberg, N Eng J Med 2012, 366: 2207; 2. Borghaei et al, Eur J Pharmacol 2009, 625: 41. Regulation der T-Zell-Aktivität: Checkpoint-Moleküle Aktivierende Rezeptoren Inhibierende Rezeptoren CTLA-4 CD28 PD-1 OX40 TIM-3 CD137 LAG-3 Aktivierung Immunsystem Adapted from Mellman I, et al. Nature. 2011:480;481–489; Pardoll DM. Nat Rev Cancer. 2012;12:252–264. Neues Therapeutisches Konzept: Blockade der CTLA-4- und PD-1-Checkpoint-Signalwege Mikroumgebung des Tumors Lymphknoten Aktivierung (Zytokine, Lyse, Proliferation, Migration zum Tumor) MHC TCR TCR Dendritische B7 CD28 Zelle B7 CTLA-4 +++ +++ +++ T-Zelle --- Anti-CTLA-4 MHC T-Zelle PD-1 PD-L1 --- Tumorzelle Anti-PD-1/PD-L1 PD-1 PD-L2 --Anti-PD-1 CTLA-4-Signalweg CTLA-4 reguliert die Amplitude der frühen Aktivierung von naiven und Memory T-Zellen. Wolchock et al, J Clin Oncol 2013 ASCO Annual Meeting Abstracts 31:15_suppl PD-1-Signalweg PD-1 begrenzt die T-Zell-Aktivierung in der Peripherie während einer Entzündungsreaktion. Die Immunonkologie: Proof of Concept Langzeitdaten Ipilimumab von 1.861 Melanompatienten (8 Ph. II-, 2 Ph. III-, 2 Ph. IV-Studien) Wahrscheinlichkeit Gesamtüberleben • 1.0 0.9 0.8 0.7 Medianes OS, Monate (95% KI): 11,4 (10,7–12,1) 3-Jahres OS-Rate, % (95% KI): 22 (20–24) 0.6 0.5 0.4 0.3 0.2 0.1 Ipilimumab Zensiert 0.0 0 12 24 36 48 60 72 84 96 108 120 120 26 15 5 0 Monate Patienten unter Progressionsrisiko Ipilimumab 1.861 839 370 254 192 170 Schadendorf et al, annual presentation at ECCO/ESMO 2013, abstract # 24LBA Die Immunonkologie und „Hallmarks of Cancer“ Adapted from Hanahan et al, Cell 2011; 44: 646 Immuntherapeutische Ansätze am Beispiel Lungenkrebs Immuntherapie Passiv (adoptiv) Aktiv Zielt auf den Tumor; Immun-basierter Mechanismus Zielt auf das Immunsystem direkt Antigenabhängig Antigenunabhängig Verstärkt die Funktion der Immunzelle Therapeutische Vakzine Moduliert die TZell Funktion Zytokine GSK1572932A TG4010 Belagenpumatucel-L Tergenpumatucel-L Racotumomab Stimuvax CIMAvax Antitumorale monoklonale Antikörper Adoptiv Bavituximab EGFR Inhibition Adoptiver Zelltransfer Immun-Onkologie (I-O) CTLA-4-Inhibition PD-1-Inhibition PD-L1-Inhibition CTLA-4 = cytotoxic T-lymphocyte antigen-4; PD-1 = programmed death-1; PD-L1 = programmed death ligand-1 www.clinicaltrials.gov accessed 26 March 2014; NCCN Guidelines ®. NSCLC. V2.2013; Peters et al. Ann Oncol. 2012;23:vii56 Klinische Entwicklung: Immun-Checkpoint-Inhibitoren – PD-1-/PD-L1-Signalweg Target Antibody a Nivolumab (BMS-936558) PD-1 Pembrolizumab (MK-3475) Pidilizumab (CT-011) MEDI-4736 PD-L1 MPDL3280A MSB0010718C Development Stage Phase 1-3: multiple tumors (melanoma, RCC, NSCLC, HNSCC, GBM, Hodgkin, others) Phase 1/2: multiple tumors Phase 3: NSCLC Approved: melanoma Phase 1/2: multiple tumors Phase 1/2: multiple tumors Phase 2: CRC, HNSCC Phase 3: NSCLC Phase 1/2: multiple tumors Phase 2: RCC, bladder Phase 3: NSCLC Phase 1/2: multiple tumors CRC = colorectal cancer; GBM = glioblastoma multiforme; HNSCC = head and neck squamous cell carcinoma; RCC = renal cell carcinoma a List incomplete www.clinicaltrials.gov. Accessed March 25, 2015. Phase I-Studie Nivolumab Monotherapie: Design der NSCLC-Kohorte (CA209-003) • Phase 1 study of the safety, antitumor activity, and pharmacodynamics of nivolumab in patients with advanced solid tumors1 • Nivolumab 0,1 to 10 mg/kg Q2W for a maximum of twelve 8-week treatment cycles (2 years); expansion cohorts for select tumor types2 • 1–5 previous therapies1 • In the 129 patients with NSCLC – 42% had squamous and 57% had non-squamous histology – 54% received ≥3 prior therapies2 IV = intravenous; Q2W = every 2 weeks. 1. Topalian SL, et al. N Engl J Med. 2012;366:2443-2454. 2. Brahmer JR, et al. Presented at ASCO 2013. Abstract 8030. Nivolumab 1 mg/kg IV Q2W (n=33) Eligible patients with NSCLC randomized between 3 nivolumab dose levels Nivolumab 3 mg/kg IV Q2W (n=37) (n=129) Nivolumab 10 mg/kg IV Q2W (n=59) Der anti-PD-1-AK Nivolumab bei fortgeschrittenen Tumorerkrankungen: Phase I – CA209-003 Tumor Type NSCLC Squamous Nonsquamous MEL RCC ORR n/N (%) [95% CI] 22/129 (17,1) [11,0, 24,7] 9/54 (16,7) [17,9, 29,3] Median Duration of Response, wk (range) 74,0 (6,1+, 133,9+) NR (16,1, 133,9+) Stable Disease, n/N (%) [95% CI] ≥ 24 wk 13/129 (10,1) [5,5, 16,6] 8/54 (14,8) [6,6, 27,1] Median PFS, mo [95% CI] 2,3 [1,9, 3,7] 3,7 [1,8, 7,2] 13/74 (17,6) [9,7, 28,2] 63,9 (6,1+, 74,0+) 5/74 (6,8) [2,2, 15,1] 2,0 [1,8, 3,6] 33/107 (30,8) [22,3, 40,5] 10/34 (29,4) [15,1, 47,5] 104,0 (18,4, 117,0+) 56,1 (36,6, 126,7+) 7/107 (6,5) [2,7, 13,0] 9/34 (126,5) [12,9, 44,4] 3,7 [1,9, 9,1] 7,3 [3,7, 12,9] Hodi et al, Poster presentation at ECC 2013:abstract 880 Nivolumab beim fortgeschrittenen NSCLC: Überleben Phase I CA209-003 (mehrfach vorbehandelte Patienten) 100 OS rate, % (95% CI) 90 Censored 80 70 Group Died/Treated 1 mg/kg 3 mg/kg 10 mg/kg 99/129 23/37 50/59 Median OS, mo (95% CI) 9,9 (7,8, 12,4) 14,9 (7,3,30,3) 9,2 (5,2,12,4) 1 Year 2 Years 3 Years 42 (33, 50) 56 (38,71) 38 (26, 50) 24 (17, 33) 42 (24,58) 20 (11, 31) 18 (11, 25) 27 (12, 43) 14 (7, 25) OS (%) 60 50 2-year OS = 42% 40 3-year OS = 27% 30 20 10 0 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 Months Since Initiation of Treatment Pts at Risk Nivolumab 1 mg/kg 33 26 21 16 9 7 6 6 4 4 4 3 1 1 0 0 0 0 0 0 0 0 0 Nivolumab 3 mg/kg 37 34 26 21 17 14 13 12 11 9 9 7 5 2 1 1 1 1 1 1 1 1 0 Nivolumab 10 mg/kg 59 51 35 29 22 16 14 12 11 10 9 9 6 4 2 2 2 1 1 0 0 0 0 Gettinger et al, CMSTO 2014 Der anti-PD-1-AK Pembrolizumab beim fortgeschrittenen NSCLC: Phase I-Studie – Wirksamkeit RECIST v1.1, Independent Review irRC, Investigator Review Subgroup n ORR, n (%) [95% CI] Median PFS, wk (95% CI) n ORR,* (%), [95% CI] Median PFS, wk (95% CI) Median OS, wk (95% CI) All 38 9 (24%) [11%, 40%] 9,1 (8,3, 17,4) 33 7 (21%) [9%, 39%] 9,7 (7,6, 17) 51 (14, NR) Non-squamous 31 7 (23%) [10%, 41%] 9,1 (8,3, 17,0) 26 4 (16%) [4%, 35%] 10,3 (7,6, 17) 35 (14, NR) Squamous 6 2 (33%) [4%, 78%] 23,5 (2,7, NR) 6 2 (33%) [4%, 78%] 15,2 (1,4, NR) NR (2,7, NR) Patients with measurable disease on baseline imaging and an evaluable tumor specimen for PD-L1 Score ≥ potential cut point 9 6 (67%) [30%, 93%] – 7 4 (57%) [18%, 90%] – – Score < potential cut point 24 1 (4%) [0%, 21%] – 22 2 (9%) [1%, 29%] – – Garon et al, WCLC 2013, #2416 Einfluss der Histologie – Wirksamkeit der Anti-PD-1-/PD-L1-Antikörper Nivolumab MPDL3280A Squamous NS S NS NS NS S Nonsquamous NS On study, on treatment S 0 16 32 48 64 80 96 Time (week) On study, post treatment Duration of response on study NS Treatment discontinued Ongoing response NS Ongoing response Time to response NS First response Response duration after discontinuation NS First PD 112 128 144 160 0 6 12 18 24 30 36 42 48 Time (weeks) Adapted from Brahmer JR, et al. Mini-Oral presentation at WCLC 2013. J Thorac Oncol. 2013;8(Suppl 2):abstract: MO18.03; Horn L, et al. Mini-Oral presentation at WCLC 2013. J Thorac Oncol. 2013;8(Suppl 2):abstract: MO18.01. 54 60 66 72 78 KEYNOTE-001: Geschätzte Überlebenskurve (Kaplan-Meier) PFS (Recist v1.1, Central Review) • Treatment naive – Median PFS: 27 weeks (95% CI, 14–45) – 24-week PFS: 51% • Previously treated – Median PFS: 10 weeks (9,1–15,3) – 24-week PFS: 26% Analysis cutoff date: March 3, 2014 Garon et al, Oral presentation at ESMO 2014 OS • Treatment naive – Median OS: NR (95% CI, NE-NE) – 6-month OS: 86% • Previously treated – Median OS: 8,2 months (7,3 - NR) – 6-month OS: 59% Klinisches Ansprechen nach PD-L1-Immunohistochemie Anti PD-1 MK-3475 ORR n/N (%) All patients 21% Anti PD-L1 Nivolumab ORR n/N (%) 22/129 (17,1%) MEDI4736 ORR n/N (%) MPDL3280A ORR n/N (%) 9/58 (16%) 12/53 (23%) PD-L1 Status (evaluable pts.) Positive 37/159 (23%) 5/31 (16%) 5/20 (25%) 8/26 (31%) Negative 3/35 (9%) 4/32 (13%) 1/29 (3%) 4/20 (20%) Offene Fragen • Unterschiedliche Gewebsentnahmezeiten • Unterschiedliche Antikörper und Assays • Unterschiedliche IHC-Kriterien Horn L, J Thorac Oncol 2013; 8 (Suppl 2), #MO18.01; Brahmer JR, J Thorac Oncol 2013; 8 (Suppl2), #MO1803; Antonia SJ, J Thorac Oncol 2013 (Suppl 2), #P2 11-034; Garon E, ASCO 2014; #8020; Brahmer J, ASCO 2014, #8021 Pembrolizumab – Phase I-Studie 100 90 80 70 60 50 40 30 20 10 0 Strong Weak Negative 0 n at risk Strong Weak Negative OS 8 16 24 32 40 Overall Survival, % Progression-Free Survival, % PFS (Recist v1.1, Central Review) 48 28 43 30 18 17 15 17 12 7 Strong Weak Negative 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Time, months Time, weeks 44 53 49 100 90 80 70 60 50 40 30 20 10 0 9 6 1 6 0 0 3 0 0 44 43 38 38 34 32 30 27 21 18 9 53 51 48 40 34 31 26 22 18 11 8 49 42 38 34 29 26 21 14 8 6 4 8 7 2 5 5 0 5 5 0 • PFS was longer in patients with PD-L1 strong-positive versus PD-L1 weak-positive/ negative tumors (HR, 0.52; 95% CI, 0.33-0.80) • OS was longer in patients with PD-L1 strong-positive versus PD-L1 weak-positive/ negative tumors (HR, 0.59; 95% CI, 0.35-0.99) a Evaluable patients were those patients in the training set with evaluable tumor PD-L1 expression. Strong PD-L1 positivity defined as staining in ≥50% of tumor cells, and weak PD-L1 positivity as staining in 1-49% of tumor cells. Negative staining is no PD-L1 staining in tumor cells. Analysis cutoff date: March 3, 2014; Garon et al, Oral presentation at ESMO 2014 4 4 0 Korrelation zwischen PD-L1-Status und klinischem Outcome: Ein potentieller prädiktiver Biomarker? Daten mit PD-1-Checkpoint-Inhibitoren weisen darauf hin, dass die PD-L1Expression im Tumor signifikant mit einem verbesserten Ansprechen und Überleben assoziiert ist1….. ABER Patienten mit PD-L1-negativen Tumoren sprechen auch auf die Therapie an2,3… 1. Garon EB, et al. Presented at ESMO 2014. Abstract LBA 43. 2. Gettinger SN, et al. Presented at CMSTO 2014. Poster 170. 3. Ramalingam S, et al. Presented at CMSTO 2014. Abstract 3462 NSCLC – Zulassungsrelevante Studien mit Nivolumab STUDY PRIMARY ENDPOINT STATUS PUBLICATION Nivolumab ORR ongoing not recruiting Rizvi N.A,, Wolf J., Grohé C., Huber R.M., et al. The Lancet Oncology 16.3 (2015): 257-265. Allcomer Nivolumab vs. Docetaxel OS ongoing not recruiting 2L+ Allcomer Nivolumab vs. Docetaxel OS ongoing not recruiting 1L PD-L1+ Nivolumab vs. ICC PFS ongoing not recruiting LINE ALLCOMER PD-L1+ PHASE POPULATION CA209-063 2 NSCLC squamous 3L+ Allcomer CA209-017 3 NSCLC squamous 2L CA209-057 3 NSCLC non-squamous CA209-026 3 NSCLC squamous non-squamous DESIGN ORR = Objective Response Rate, OS = Overall Survival, PFS= Progression Free Survival, ICC = Investigator's Choice Chemotherapy www.clinicaltrials.gov Nivolumab Phase II CA209-063: Studiendesign Population Stage IIIB/IV NSCLC squamous • ≥2 prior systemic therapies • ECOG 0–1 Treatment Endpoints Primary: • Confirmed ORR (IRC assessed) Nivolumab 3 mg/kg IV Q2W* n=117 Secondary: • Confirmed ORR (investigator assessed) Exploratory: • Safety and tolerability • PFS/OS • PD-L1 expression and efficacy Title: Study of Nivolumab (BMS-936558) in Subjects With Advanced or Metastatic Squamous Cell Non-Small Cell Lung Cancer Who Have Received At Least Two Prior Systemic Regimens (CheckMate 063) ORR = Objective Response Rate, IRRC = Independent Radiology Review Committee OS = Overall Survival, PFS= Progression Free Survival * until disease progression, discontinuation due to toxicity, withdrawal of consent or the study ends www.clinicaltrials.gov CA209-063: Ansprechen und Überleben (Größte Reduktion der Targetläsion)a Best Reduction from Baseline in Target Lesion (%) 100 Alive Expired Confirmed responders 75 50 25 0 -25 -50 -75 n=95 response-evaluable -100 Patient • 8 confirmed PRs in patients with rapid progression on prior therapy • Among all treated patients; 13% continuing treatment and 24% received subsequent therapy – Most frequent therapies were gemcitabine (10%), docetaxel (4%), and vinorelbine (4%) – No patients received subsequent immunotherapy a Based on July 2014 DBL; 22/117 treated patients are not displayed due to lack of evaluable on-study assessments; 18/22 expired; Dashed horizontal reference line indicates the 30% reduction consistent with a RECIST v1.1 response; Ramalingam et al. CMSTO 2014 CA209-063: Klinische Aktivität von Nivolumab Nivolumab 3 mg/kg ORR, % (n) [95% CI] Disease control rate, % (n) Median DOR, months (range) IRC Assessed (per RECIST v1.1)a 15 (17) [9, 22] 40 (47) NR (2+, 12+) Ongoing responders, % (n) 76 (13) Median time to response, months (range) 3 (2, 9) PFS rate at 1-year, % (95% CI) 20 (13, 29) Median PFS, months (95% CI) 2 (2, 3) • Investigator-assessed ORR was 13% (95% CI, 7, 20) – Concordance between IRC and investigator assessed responders was 92% (based on March 2014 DBL) a July 2014 DBL NR = not reached; DOR = duration of response; ORR = objective response rate; PFS = progression free survival Ramalingam et al. CMSTO 2014 CA209-063: Ansprechmustera Percent Change in Baseline (%) 100 1st Occurrence of new lesion PR Patients still on treatment 75 50 25 0 -25 -50 -75 -100 0 6 12 18 25 30 36 42 48 54 60 Time Since First Treatment (Weeks) • 4 patients with non-conventional responses not reported as IRC-assessed responders – 3 alive (OS: 12+, 13+, 14+ months) a Based on July 2014 DBL Ramalingam et al. CMSTO 2014 66 CA209-063: Gesamtüberleben (OS): Alle behandelten Patientena Overall Survival (%) 100 Median OS, months (95% CI) 8,2 (6, 11) 90 1-year OS rate, % (95% CI) 41 (32, 50) 80 Number of events 72/117 70 Median OS = 8,2 months 60 50 1-year OS = 41% 40 30 20 10 0 0 Number of Patients at Risk Nivolumab 117 3mg/kg • 3 6 9 12 15 18 28 5 0 Overall Survival (Months) 93 68 51 Median follow-up for survival: 8 months (range 0–17 months) a Based on July 2014 DBL; Symbols represent censored observations Ramalingam et al. CMSTO 2014 CA209-063: Therapiebedingte unerwünschte Ereignisse in ≥10% der Patientena Nivolumab 3 mg/kg (n = 117) Any Grade Grade 3–4 74 17 Fatigue 33 4 Decreased appetite 19 0 Nausea 15 0 Asthenia 12 0 Rash 11 1 Diarrhea 10 3 Total patients with an event,b % • • • • 85% of patients received at least 90% of their planned dose intensity 12% discontinued treatment due to study drug toxicity (4% pneumonitis) Grade 3 treatment-related pneumonitis was reported in 4 patients (3%); one additional grade 3 case occurred between 30–100 days after last nivolumab dose 62% had expired at time of analysis (54% PD and 2% study drug toxicity) – Two treatment-related deaths (1 hypoxic pneumonia and 1 ischemic stroke) occurred in patients with multiple comorbidities and concurrent PD a July 2014 DBL; b Includes events reported between first dose and 30 days after last dose of study therapy. Of the adverse events included in the table, no events were grade 5 Ramalingam et al. CMSTO 2014 Studiendesign: CA209-017 und CA209-057 Phase 3 CA209-017 Stage IIIb/IV squamous cell NSCLC 2L Phase 3 CA209-057 www.clinicaltrials.gov Stage IIIb/IV nonsquamous cell NSCLC 2/3L Nivolumab 3 mg/kg IV q2w OS Docetaxel 75 mg/m2 IV q3w OS Nivolumab 3 mg/kg IV q2w OS Docetaxel 75 mg/m2 IV q3w OS http://www.bms.com/news/press_releases/pages/default.aspx Fazit: PD-1-Inhibition beim NSCLC • Immun-Checkpoint-Inhibitoren zeigen ermutigende Ergebnisse in klinischen Studien (Phase 1-3) • Eindrucksvolles Ansprechen und Überleben bei vorbehandelten Patienten mit Adeno- und Plattenepithelkarzinomen • Ein Teil der Patienten zeigt möglicherweise ein Langzeitüberleben • Toxizität mäßig und beherrschbar • Mehrere Substanzen in klinischer Entwicklung • PD-L1 erster prädiktiver Biomarker, aber auch PD-L1-negative Patienten profitieren • Ausblick: Optimierung durch Kombinationstherapien Die Immunonkologie als fest verankertes Behandlungskonzept in der Onkologie Operation Immunonkologie Strahlentherapie Chemo- & zielgerichtete Therapien DeVita et al, N Eng J Med 2012; 366: 2207; Borghaei et al, Eur J Pharmacol 2009; 625: 41 Stand: April 2015 Bitte beachten Sie, dass sehr bald neue Daten und Updates der hier vorgestellten Studien folgen!