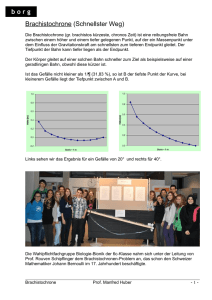
Gestaltung des Titelblattes
Werbung

Immunphänotypische Charakterisierung CD11c-positiver Zellen des Gehirns im direkten Vergleich zu CD11c-positiven Zellen von Lunge, Leber und Milz Dissertation zur Erlangung des akademischen Grades Dr. rer. med. an der Medizinischen Fakultät der Universität Leipzig eingereicht von: Kerstin Immig geb. am 11.08.1983 in Leipzig angefertigt an der Medizinischen Fakultät der Universität Leipzig am Institut für Anatomie Betreuer: Herr Prof. Dr. med. Ingo Bechmann Beschluss über die Verleihung des Doktorgrades vom: 23.03.2016 2 Bibliografische Beschreibung: Immig, Kerstin Immunphänotypische Charakterisierung CD11c-positiver Zellen des Gehirns im direkten Vergleich zu CD11c-positiven Zellen von Lunge, Leber und Milz Universität Leipzig, Dissertation 71 S.1, 101 Lit.2, 3 Abb., 2 Publikationen Referat Bei der vorliegenden Arbeit handelt es sich um eine experimentell durchgeführte Charakterisierung von CD11c-positiven Zellen des Gehirns im direkten Vergleich zu CD11cpositiven Zellen aus Lunge, Leber und Milz. Mittels Konfokal- und Fluoreszenzmikroskopie wurde die Existenz von intraparenchymalen Zellen nachgewiesen, welche den Zellmarker für Dendritische Zellen CD11c exprimieren. Durch die Etablierung einer einheitlichen Isolierungsmethode von CD11c-positiven mononukleären Zellen aus dem Gehirn, Milz, Lunge und Leber, war es uns möglich, diese mittels Durchflusszytometrie, auf die Expression wichtiger Marker für mononukleäre Zellen zu untersuchen und phänotypisch miteinander zu vergleichen. Durch diese Zellanalysen zeigten wir, dass CD11c-positive Zellen des Gehirns sowohl aufgrund ihrer spezifischen CD45-Expression, als auch durch die Expression von CD11b einen Mikrogliaphänotyp aufwiesen. Dabei konnten wir beobachten, dass CD11cpositive Zellen aus dem Gehirn einzigartig in der Eigenschaft ihrer geringen Major Histocompatibility Complex (MHC)-II-Expression sind. Mit Hilfe einer transgenen Mauslinie, welche unter dem Promotor von MHC-II das grün-fluoreszierende Protein (GFP) exprimiert, konnten wir nachweisen, dass Mikroglia selbst in der Umgebung von MHC-II-positiven Zellen, kultiviert auf Schnittkulturen der Milz, ihre MHC-II-Negativität behalten. Im Vergleich dazu adaptierten sich MHC-II-positive Splenozyten, kultiviert auf Schnittkulturen vom Hippocampus, an die neue Umgebung und verringerten die Expression von MHC-II. Unsere Daten lassen also die Schlussfolgerung zu, dass sich CD11c-positive Mikroglia hinsichtlich ihrer Expression von MHC-II intrinsisch von CD11c-positiven Zellen anderer Organe unterscheiden. Ebenso scheinen auch lokale Faktoren im Gehirn dazu beizutragen, die Expression von MHC-II unter physiologischen unterdrücken. ________________ 1 Seitenzahl 2 Zahl insgesamt der im Literaturverzeichnis der Einleitung ausgewiesenen Literaturangaben Bedingungen wirkungsvoll zu 3 Inhaltsverzeichnis 1 Einleitung 4 1.1 Das Gehirn als immunprivilegiertes Organ 4 1.1.1 Das Immunprivileg und der fehlende lymphatische Abfluss 4 1.1.2 Das Immunprivileg und die Kompartimentierung im Gehirn 5 1.1.3 Das Immunprivileg und der afferente und efferente Arm des Immunsystems 6 1.1.4 Das Immunprivileg und die Bluthirnschranke 6 1.1.5 Das Immunprivileg und das Fehlen von Dendritischen Zellen im Gehirn 9 1.2 Neuroinflammation 9 1.2.1 Multiple Sklerose 11 1.3 Myeloide Zellen des ZNS 13 1.3.1 Dendritische Zellen 14 1.3.2 Makrophagen 15 1.3.3 Mikroglia 15 1.4 CD11c als Marker für Dendritische Zellen 17 2 Fragestellung 19 3 Literaturverzeichnis der Einleitung 20 4 Publikationen 30 4.1 CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system 30 4.2 CD11c-positive cells from brain, spleen, lung and liver exhibit site-specific immune phenotypes and plastically adapt to new environments 45 5 Zusammenfassung 61 6 Summary 65 7 Danksagung 68 8 Erklärung über die eigenständige Abfassung der Arbeit 69 9 Curriculum vitae 70 4 1 Einleitung 1.1 Das Gehirn als immunprivilegiertes Organ Die verzögerte oder unterdrückte Reaktion des angeborenen und adaptiven Immunsystems, zum Beispiel auf Fremdantigene eines Virus oder allogenetische Transplantate, bezeichnet man als Immunprivileg und stellt somit eine besondere immunologische Situation dar. Immunprivilegierte Orte im Körper sind zum Beispiel die vordere Augenkammer, der Hoden oder die Plazenta, aber auch das Gehirn zählt zu den immunprivilegierten Organen. Der Begriff „Immunprivileg“ wurde erstmals aufgrund von frühen Transplantationsexperimenten von Billingham und Boswell verwendet. Deren Definition des Immunprivilegs: „immunologically privileged, i.e., grafts transplanted to them are in some way partially or fully exempted from the normal rigors imposed by their histoincompatible status.”, beschreibt die Toleranz gegenüber Transplantaten (Billingham und Boswell, 1953). Schon in den zwanziger Jahren des letzen Jahrhunderts wurde von Shirai eine immunologische Barriere zwischen dem Gehirn und dem peripheren Immunsystem vermutet. Er beobachtete ein starkes Tumorwachstum, nachdem er Tumorzellen aus Ratten in das Hirnparenchym von Mäusen transplantiert hatte. Bei einer Transplantation von Tumorzellen aus Ratten außerhalb des Gehirns, in Muskelgewebe oder in die Haut, beobachtete er kein Wachstum (Shirai, 1921). Ebenfalls kam es zu keinem Tumorwachstum bei einer simultanen Transplantation des Tumorgewebes in das Hirnparenchym und in die Milz (Murphy und Sturm, 1923). Auch Medawar zeigte im Kaninchen, dass es zu keiner Abstoßungsreaktion von Hauttransplantaten im Hirnparenchym und in der vorderen Augenkammer kam. Erst als er die Haut zusätzlich in die Bauchhaut transplantierte, konnte er eine immunologische Reaktion gegen die Transplantate beobachten. Daraus schlussfolgerte er, dass Antigene im Gehirn und in der vorderen Augenkammer einer Immunantwort unterliegen können, aber sie nicht selbst auslösen (Medawar, 1948). 1.1.1 Das Immunprivileg und der fehlende lymphatische Abfluss Nachdem man das Phänomen des Immunprivilegs im zentralen Nervensystem (ZNS) beobachtet hatte, wurden verschiedene Theorien entwickelt, dies zu erklären. Aufgrund seiner oben beschriebenen Beobachtungen schlussfolgerte Medawar das Fehlen eines lymphatischen Abfluss aus dem Hirnparenchym und in der vorderen Augenkammer (Medawar, 1948). Später zeigte man, dass eine Verbindung zwischen dem Gehirn und dem lymphatischen System existiert. So besteht eine Verbindung über die Zerebrospinalflüssigkeit, die arachnoidalen Villi, das durale Sinusblut und den Subarachnoidalraum, entlang der olfaktorischen Nerven durch die Lamina cribrosa zur nasalen Submukosa (Andres et al., 5 1987; Cserr und Knopf, 1992; Ichimura et al., 1991; Kida et al., 1993). Von der nasalen Submukosa besteht ein Zugang zu den terminalen Lymphgefäßen, über welche Substanzen aus dem Gehirn über einen aktiven oder passiven Transport zu den Lymphknoten gelangen können (Cserr und Knopf, 1992). 1.1.2 Das Immunprivileg und die Kompartimentierung im Gehirn Das Gehirn unterteilt sich in das Parenchym, den Hirnhäuten und den ventrikulären Räumen mit dem Plexus choroideus und der Zerebrospinalflüssigkeit (Abbildung 1) (Galea et al., 2007). Das Immunprivileg ist jedoch nicht in allen diesen Kompartimenten nachweisbar. So beobachtete man eine Abstoßung von Maustumorgewebe, wenn dies in die Hirnventrikel von Ratten transplantiert wurde. Injizierte man das Tumorgewebe direkt in das Hirnparenchym, konnte keine Immunantwort ausgelöst werden (Murphy und Sturm, 1923). Auch allogenetische Transplantate riefen eine Abstoßungsreaktion hervor, wenn diese in die Ventrikel injiziert wurden (Mason et al., 1986). Ähnliche Beobachtungen wurden nach Injektionen von bakteriellem und viralem Material in die Ventrikel gemacht. So konnte nur eine Immunantwort bei einer Injektion in die Ventrikel ausgelöst werden, jedoch nicht bei einer Transplantation in das Hirnparenchym (Matyszak und Perry, 1998; Stevenson et al., 1997). Abbildung 1: Überblick (Galea et al., 2007) über die Kompartimente des Gehirns Die verminderte Immunreaktion auf Fremdantigene im Hirnparenchym wurde auch für die angeborene Immunantwort gezeigt. So führte eine intraventrikuläre Injektion von Lipopolysacchariden zu einer Rekrutierung von neutrophilen Granulozyten und Monozyten innerhalb von 2 h, ähnlich wie sie bei einer subkutanen Injektion beobachtet werden konnte. Wurde jedoch die gleiche Dosis in das Hirnparenchym injiziert, konnte keine Rekrutierung dieser Zellen beobachtet werden (Andersson et al., 1992). 6 1.1.3 Das Immunprivileg und der afferente und efferente Arm des Immunsystems Der afferente Arm einer Immunantwort umfasst den Transport von Antigenen zu den Lymphknoten und die Antigenpräsentation für naive T-Zellen sowie T-Gedächtniszellen. Daraufhin erfolgt eine Aktivierung und Proliferation der antigenspezifischen T-Zellen (Galea et al., 2007). Die für die Aktivierung notwendigen Antigene können aktiv mit Hilfe von Zellen, den antigenpräsentierenden Zellen, zu den Lymphknoten transportiert werden. Ein anderer Weg ergibt sich aus den sogenannten „löslichen“ Antigenen, welche vom perivaskulären Raum in die Subarachnoidalräume und, wie bereits oben beschrieben, entlang des Nervus olfaktorius durch die Lamina cribrosa gelangen (Galea et al., 2007). Von dort erreichen die Antigene über die nasale Submukosa die drainierenden Lymphbahnen (Ichimura et al., 1991). Der efferente Arm einer Immunantwort des ZNS beschreibt den Zufluss von peripheren Immunzellen, wie zum Beispiel Monozyten, neutrophile Granulozyten und T-Zellen zum Gehirn (Cserr und Knopf, 1992; Galea et al., 2007). Unter gesunden Bedingungen ist diese Migration in das Hirnparenchym stark reguliert. So wurde gezeigt, dass infiltrierende T-Zellen zur Apoptose gezwungen werden können, welche über den Todesliganden FasL initiiert wird. Die Expression von FasL konnte auf Neuronen, Mikroglia der weißen Substanz und Astrozyten, welche in der Nähe von Blutgefäßen lokalisiert sind, nachgewiesen werden (Bechmann et al., 1999). Weiterhin scheint die geringe Expression von Major Histocompatibility Complex (MHC)-II ein limitierender Faktor für die MHC-II-abhängige Erkennung des spezifischen Antigens im Gehirn zu sein (Perry, 1998). Auch die Expression der co-stimulatorischen Moleküle CD80 und CD86 hat einen wichtigen Einfluss auf die MHCII-abhängige Antigenpräsentation. So wurde beschrieben, dass CD80 die Proliferation inflammatorischer T-Helfer-Zellen fördert, wohingegen CD86 die Proliferation schützender THelfer-Zellen unterstützt und eine Toleranz im Gehirn gegenüber dem Antigen entwickelt werden konnte (Kuchroo et al., 1995). Am Beispiel der Experimente von Medawar als auch von Matyszak und Perry wurde gezeigt, dass eine Immunantwort im Gehirn ausgelöst werden kann, wenn die Transplantate in das Gehirn als auch in die Haut verpflanzt wurden (Matyszak und Perry, 1996; Medawar, 1948). Daraus schlussfolgernd gibt es einen Zufluss von peripheren Immunzellen in das Gehirn, welcher jedoch streng reguliert wird. 1.1.4 Das Immunprivileg und die Bluthirnschranke Die Bluthirnschranke (BHS) beschreibt den fehlenden Übergang bestimmter Stoffe vom Blut in das ZNS. Erste Beobachtungen wurden von Paul Ehrlich im Rahmen von Studien über das Sauerstoffbedürfnis verschiedener Organe im Jahre 1885 beschrieben, als er bei der Injektion von hydrophilen Farbstoffen beobachtete, dass sich das Gehirn und das Rückenmark nicht anfärben ließen (Ehrlich, 1885). In einer späteren Arbeit wurden die 7 Kapillarwände als eine der Barrieren identifiziert, welche den Übertritt bestimmter Stoffe in das ZNS verhinderten (Lewandowski, 1900). Später prägte Goldmann den Begriff der physiologischen Grenzmembran. So beschrieb er, dass sich der hinter der Plazenta liegende Fetus sowie fett- und glykogenspeichernde Zellen nicht von bestimmten Farbstoffen anfärben ließen. Daraus schlussfolgerte er, dass physiologische Grenzmembranen und deren Fähigkeit zur Phagozytose diese Gewebe von anderen abschirmen. In weiteren Untersuchungen stellte er jedoch fest, dass das Gehirn nicht komplett von der Umgebung abgeschirmt ist und sich Zellen des Plexus choroideus, der weichen Hirnhäute und entlang der perivaskulären Räume mit Farbstoffen anfärben ließen. Die perivaskulären Räume interpretierte er als Lymphräume. Da die Farbstoffe in Zellen eingeschlossen waren, mutmaßte er, dass diese Zellen zur Phagozytose und Migration fähig sind. Diese perivaskulären Phagozyten wurden seither mehrfach beschrieben (Angelov et al., 1998; Fabriek et al., 2005). Weiterhin vermutet man, dass einige der perivaskulären Zellen zur Antigenpräsentation fähig sind und daher eine entscheidende Funktion für den Ausbruch autoimmuner Erkrankungen haben (Greter et al., 2005; Hickey und Kimura, 1988). Mit Hilfe von elektronenmikroskopischen Studien konnte in einer weiteren Arbeit gezeigt werden, dass das morphologische Korrelat der BHS bestimmte Verschlusskontakte zwischen benachbarten Endothelzellen in Form von Tight junctions (TJ) sind. Die Autoren schlussfolgerten, dass diese den Durchtritt bestimmter Stoffe aus der Blutbahn in das Gehirn zwischen benachbarten Endothelzellen verhindern (Reese und Karnovsky, 1967). Abbildung 2: Topografie der neurovaskulären Einheit (Krueger und Bechmann, 2010) Weitere Arbeiten zeigten zudem, dass nicht nur das Endothel, sondern auch benachbarte Zellpopulationen gleichermaßen an der Aufrechterhaltung der Funktion der BHS beteiligt sind und prägten daher den Begriff der neurovaskulären Einheit (Abbildung 2). Diese besteht 8 aus drei Kompartimenten: der Gefäßwand, dem perivaskulären Raum und dem juxtavaskulären Neuropil. Alle drei Kompartimente sind durch Basalmembranen voneinander getrennt (Bechmann et al., 2001a). Die Gefäßwand kann aus Endothelzellen, Perizyten und, außerhalb des Kapillarbettes, glatten Muskelzellen bestehen. Der perivaskuläre Raum, auch Virchow-Robin-Raum genannt, enthält perivaskuläre Flüssigkeit und perivaskuläre Zellen (Zellen der weichen Hirnhäute und perivaskuläre Makrophagen). Das dritte Kompartiment besteht aus dem Neuropil bzw. dem Hirnparenchym. Dieses wird durch die Glia limitans begrenzt, welche sich hauptsächlich aus den Endfüßen der Astrozyten und wenigen juxtavaskulären Mikrogliazellen zusammensetzt (Bechmann et al., 2007; Krueger und Bechmann, 2010). Alle Komponenten der neurovaskulären Einheit haben einen Einfluss auf die Funktion der BHS und die Aufrechterhaltung der Homöostase im ZNS (Weiss et al., 2009). Da der Begriff der BHS parallel mit den verstärkten Untersuchungen des Immunprivilegs des Gehirns aufkam, wurde versucht das Immunprivileg mit dem Phänomen der BHS zu erklären (Barker und Billingham, 1977). Unter physiologischen Bedingungen kommt es zu einer kontrollierten Migration von Leukozyten in das Gehirn (Hickey, 2001), welche sich unter inflammatorischen Bedingungen stark erhöht (Ajami et al., 2011; Priller et al., 2001; Wekerle, 1993). Ebenso konnte bei einer Transplantation von neuronalem Mausgewebe in Rattenhirn eine Infiltration von T-Zellen und Makrophagen aus der Peripherie in das Transplantat 35 Tage nach der Transplantation beobachtet werden (Finsen et al., 1991). Das Immunprivileg lässt sich jedoch nicht vollständig mit den Beobachtungen der BHS erklären. So wurde gezeigt, dass Leukozyten hauptsächlich über postkapilläre Venulen in das Gehirnparenchym migrieren und nicht über die Kapillaren, an welchen die TJ besonders ausgeprägt sind (Raine et al., 1990). Darüber hinaus konnte eine transzelluläre Migration von Leukozyten in das Gehirn beschrieben werden (Engelhardt und Ransohoff, 2005; Wolburg et al., 2005). Weiterhin wurde belegt, dass die Infiltration von Leukozyten nicht mit dem Ort der größten Bluthirnschrankenpermeabilität korreliert (Muller et al., 2005). Im Modell einer anterograden, axonalen Degeneration konnte gezeigt werden, dass es zu keinem Einstrom der Permeabilitätsmarker Meerrettichperoxidase oder Evans Blau in das Neuropil kam (Jensen et al., 1997). Beide Substanzen sind häufig benutzte Marker um die Durchlässigkeit der BHS bzw. von Gefäßen zu kontrollieren. Zusätzlich konnte im gleichen Tiermodell gezeigt werden, dass es unter diesen inflammatorischen Bedingungen jedoch zu einer Infiltration von TZellen kommt (Bechmann et al., 2001b). Ebenfalls konnte gezeigt werden, dass zirkulierende Monozyten unter dem Einfluss einer anterograden, axonalen Degeneration im Hirnparenchym eine mikrogliaähnliche Form annehmen können (Bechmann et al., 2005). Desweiteren muss man bei den Beschreibungen über die Leukozytenrekrutierung in das Gehirn beachten, dass postkapilläre Venulen vom Neuropil durch ein weiteres 9 Kompartiment, den perivaskulären Raum, getrennt sein können. Auch unter gesunden Bedingungen konnte mit der intraventrikulären Injektion von verschiedenen fluoreszierenden Farbstoffen, welche über Phagozytose aufgenommen werden, periphere Makrophagen im perivaskulären Raum in Ratten beobachtet werden, allerdings nicht im Parenchym. Auch bei der Reinjektion von markierten Makrophagen in die Schwanzvene konnte eine Migration von Makrophagen aus der Peripherie in den perivaskulären Raum gezeigt werden. (Bechmann et al., 2001a). Von daher ist der Begriff der BHS, welche den Übertritt bestimmter Stoffe vom Blut in das ZNS verhindert, nicht mit dem Mechanismus der Migration von Leukozyten aus der Peripherie in das ZNS gleichzusetzen. 1.1.5 Das Immunprivileg und das Fehlen von Dendritischen Zellen im Gehirn Eine weitere Theorie zur Erklärung des Immunprivilegs im Gehirn bezieht sich auf das Fehlen von Dendritischen Zellen (DCs) im ZNS (Sedgwick, 1995). DCs sind antigenpräsentierende Zellen, welche Antigene am Ort der Entzündung aufnehmen, zu den assoziierten Lymphknoten migrieren, um dort T-Zellen zu aktivieren. Auch diese Sichtweise wurde jedoch in Frage gestellt nachdem im gesunden Rattenhirn DCs sowohl in den Meningen als auch im Plexus choroideus gefunden wurden (Matyszak und Perry, 1996; McMenamin, 1999; McMenamin et al., 2003). Man nimmt jedoch an, dass die Anzahl von DCs im Gehirn unter gesunden Bedingungen sehr gering ist. Ein vielfach verwendeter Marker für die Erkennung von DCs ist das Integrin CD11c. Da immunhistochemische Untersuchungen und derzeit verfügbare Antikörper kaum positive Färbungen im Gehirn zeigten, war es schwer DCs unter gesunden Bedingungen im ZNS und speziell im Hirnparenchym und im perivaskulären Raum nachzuweisen. Mit der Entwicklung von transgenen Mausmodellen, welche unter dem Promotor von CD11c (itgax) das grünfluoreszierende Protein (GFP) (Jung et al., 2002) oder das gelb-fluoreszierende Protein (YFP) (Lindquist et al., 2004) exprimieren, war es nun möglich, diese Zellen unter physiologischen Bedingungen zu untersuchen und die Verteilung im Gehirn zu analysieren (Bulloch et al., 2008; Prodinger et al.,2011; Wieghöfer et al., 2014). Benutzt man also CD11c als alleinigen DC-Marker, so lässt sich das Immunprivileg des ZNS nicht mit dem Fehlen von DCs erklären, da man CD11c-exprimierende Zellen im perivaskulären Raum als auch im Parenchym beobachten konnte. 1.2 Neuroinflammation Unter inflammatorischen Bedingungen kommt es zu einem erhöhten Aufkommen von Leukozyten wie DCs, neutrophilen Granulozyten, T-Zellen und B-Zellen im Gehirn (Matyszak und Perry, 1996). Durch den perivaskulären Raum zwischen Gefäßwand und dem Neuropil ist die Leukozyteninfiltration aus der Peripherie in den postkapillären Gefäßen in zwei 10 Schritte unterteilt. Zuerst muss die innere und äußere Basalmembran der Gefäßwand durchquert werden, um in den perivaskulären Raum zu gelangen. Viele Leukozyten verbleiben auch unter inflammatorischen Bedingungen in diesem Kompartiment. Weiterhin ist der CC-Chemokin-Ligand (CCL2), welcher an den Rezeptor CCR2 bindet, wichtig für die Migration durch die Gefäßwand in den perivaskulären Raum. Neben der Aufgabe als chemischer Lockstoff für periphere Leukozyten, kann die Bindung von CCL2 an den Rezeptor eine Umverteilung der TJ-Proteine und Änderungen des Aktinzytoskelettes von Endothelzellen bewirken (Babcock et al., 2003; Semple et al., 2010; Toft-Hansen et al., 2006). Mäuse welche kein CCR2 oder CCL2 exprimierten, zeigten eine verringerte Migration von infiltrierenden Leukozyten als auch keine oder abgeschwächte Symptome bei der experimentellen Autoimmunenzephalomyelitis (EAE) (Huang et al., 2002; Semple et al., 2010). In einem zweiten Schritt muss die Glia limitans passiert werden, welche sich hauptsächlich aus den astrozytären Endfüßen, einigen juxtavaskulären Mikroglia und der angrenzenden Basalmembran zusammensetzt. Die Basalmembranen der Gefäßwand unterscheiden sich in ihrem molekularen Aufbau von der Basalmembran der Glia limitans. So enthält nur die Basalmembran der Glia limitans die Isoformen Laminin 1 und 2 (Owens et al., 2008; Sixt et al., 2001). Diese Beobachtung könnte erklären, warum infiltrierende Leukozyten die Gefäßwand durchqueren können, aber nicht die Glia limitans und im perivaskulärem Raum verbleiben. In weiteren Studien konnte gezeigt werden, dass auch die Matrixmetalloproteinasen (MMP) 2 und 9 benötigt werden, um eine Neuroinflammation auszulösen (Agrawal et al., 2006). MMP 2 und 9 besitzen eine hohe Affinität zu Dystroglykan, welches Laminin 1 und 2 verankert und so die astrozytären Endfüße mit der Basalmembran der Glia limitans verbindet. Transgene Mäuse, welche kein MMP 2 und 9 exprimierten, entwickelten keine EAE, da die Bindung von Dystroglykan durch MMP 2 und 9 verhindert wurde und es so zu keiner Leukozyteninfiltration in das Neuropil kam (Agrawal et al., 2006). In weiteren Arbeiten in der Maus konnte gezeigt werden, dass auch perivaskuläre Makrophagen eine wichtige Funktion bei der Entwicklung einer Neuroinflammation haben. Durch die Depletion der perivaskulären Makrophagen kam es nach einer Induktion einer EAE zwar zu einer Ansammlung von Leukozyten im perivaskulären Raum, aber nicht zu einer Infiltration in das Neuropil. Zudem waren keine klinischen Symptome einer EAE sichtbar (Tran et al., 1998). Die gleiche Arbeitsgruppe benutzte Pertussistoxin, um eine Entzündung in Mäusen auszulösen, welche CCL2 überexprimieren. In diesem Mausmodell kommt es durch die Injektion von Pertussistoxin zu einer Erhöhung von proinflammatorischen Zytokinen, welche dann zu einer Enzephalopathie führen. Dabei beobachteten sie eine Infiltration der Leukozyten in das Hirnparenchym. Bei einer Behandlung dieser Mäuse mit einem Matrixmetalloproteinaseninhibitor, nach der 11 Behandlung mit Pertussistoxin, konnte nur eine Leukozyteninfiltration in den perivaskulären Raum beobachtet werden, aber nicht in das Neuropil. Ebenso waren keine inflammatorischen Symptome in diesen Mäusen erkennbar (Toft-Hansen et al., 2006). Diese Arbeiten zeigen, dass MMPs, welche wahrscheinlich von perivaskulären Makrophagen oder T-Zellen gebildet werden (Agrawal et al., 2006), eine wichtige Rolle bei der Durchführung des zweiten Schrittes der Neuroinflammation und der eigentlichen Entstehung von klinischen Symptomen spielen. Des Weiteren ist in diesem Kontext zu erwähnen, dass auch andere Regulationssysteme die Leukozyteninfiltration aus dem perivaskulären Raum in das Hirnparenchym beeinflussen können. So wurde für den Todesliganden FasL gezeigt, dass dieser einen Einfluss auf die Infiltration in das Hirnparenchym hat (Bechmann et al., 1999; Sabelko-Downes et al., 1999). Wie oben beschrieben, sind Mikroglia ein wichtiger Bestandteil des Neuropils und werden auch als Makrophagen des Gehirns bezeichnet. Unter stabilen Bedingungen zeigen sie eher einen antiinflammatorischen Phänotyp. Jedoch konnte auch bei ihnen unter inflammatorischen Konditionen ein Anstieg von Zellmarkern beschrieben werden, die eher als proinflammatorisch angesehen werden (Carson et al., 1998; Matyszak und Perry, 1998). 1.2.1 Multiple Sklerose Multiple Sklerose (MS) ist mit rund 2,5 Millionen Betroffenen eine der häufigsten Autoimmunerkrankungen weltweit, welche mit einer Demyelinisierung im ZNS einhergeht (Goldmann und Prinz, 2013; Ortiz et al., 2014; Ransohoff, 2012). Die Krankheit kann das Gehirn, das Rückenmark und den Nervus opticus betreffen. Im Falle von MS ist die Autoreaktivität meistens gegen das basische Myelinprotein (MBP), das Proteolipid-Protein (PLP) oder das Myelin-Oligodendrozyten-Glykoprotein (MOG) gerichtet. Die klinischen Symptome dieser Erkrankung sind sehr heterogen und zeigen sich unter anderem in Sehstörungen, Störungen der Motorik, Störungen der Koordination, Blasen- und Darminkontinenz, Spastiken, Störungen der Sensorik, Störungen der Propriozeption und der Sprache (Ortiz et al., 2014; Steinman, 1996). Durch die Infiltration von autoreaktiven, myelinspezifischen T-Zellen in das ZNS kommt es zu einer T-Zell-vermittelten Immunantwort, welche mit einer weiteren Rekrutierung von peripheren Makrophagen und einer Aktivierung der Mikroglia einhergeht (Lassmann et al., 2012). Die Interaktion der T-Zellen mit antigenpräsentierenden Zellen im ZNS führt zu einer Expression von weiteren löslichen Faktoren wie Zytokinen, Chemokinen und Proteasen, welche eine weitere Infiltration von inflammatorischen autoreaktiven Leukozyten und die Umstrukturierung der BHS zur Folge hat (Babcock et al., 2003; Ransohoff, 2012; Semple et al., 2010; Toft-Hansen et al., 2006). Ebenso wurde in mehreren Publikationen gezeigt, dass Myelinantigene in Lymphknoten von MS-Patienten und in Tiermodellen nachweisbar waren 12 (Locatelli et al., 2012; Mutlu et al., 2007; van Zwam et al., 2009). Daraus ergab sich die Fragestellung, auf welchem Weg die Antigene die assoziierten Lymphknoten erreichen können. Hierfür werden zwei Möglichkeiten diskutiert. Zum einem ist dies der passive Transport über die Zerebrospinalflüssigkeit und zum anderen der aktive Transport über DCs. In ersten Arbeiten wurden bereits 12 h nach der Injektion von T-Zellen und Monozyten in das Areal einer entorhinalen Kortexläsion erste injizierte Leukozyten in den zervikalen Lymphknoten beobachtet (Goldmann et al., 2006; Kaminski et al., 2012). Dies unterstützt wiederum die Annahme, dass ein zellulärer Transport ZNS-spezifischer Antigene möglich sein könnte, um drainierende Lymphknoten zu erreichen. Die Überwindung der BHS ist ein wichtiger Prozess bei der Entwicklung einer Neuroinflammation. Wie bereits oben beschrieben sind MMP 2 und 9 wichtig für die Passage der Glia limitans und deren Basalmembran (Agrawal et al., 2006; Toft-Hansen et al., 2006). Diese können von T-Zellen, Makrophagen und Mikroglia gebildet werden (Agrawal et al., 2006; Correale und Villa, 2007; Ortiz et al., 2014). Auch Chemokine fördern das Passieren der BHS von T-Zellen. So spielt CCL2, wie bereits unter 1.2 beschrieben, beim Passieren des Gefäßendothels und deren Basalmembranen eine Rolle (Babcock et al., 2003; ToftHansen et al., 2006). So wurde gezeigt, dass das Chemokin CCL2 von Astrozyten und Mikroglia gebildet werden kann (Babcock et al., 2003). Der dazu gehörige Chemokinrezeptor konnte auf infiltrierenden Leukozyten, Mikroglia, Makrophagen und Endothelzellen nachgewiesen werden (Correale und Villa, 2007; Semple et al., 2010). Weiterhin werden inflammatorische Zytokine von T-Zellen, Makrophagen, Astrozyten und Mikroglia gebildet, wie zum Beispiel Interferon-ϒ, Tumornekrosefaktor-α, Interleukin-23 und Interleukin-17. Diese können neurotoxisch wirken und haben eine weitere Rekrutierung inflammatorischer Leukozyten aus der Peripherie zur Folge (Ortiz et al., 2014; Ransohoff, 2012). Grundsätzlich wird die MS in zwei Subtypen unterteilt. Zum einen gibt es die langsamere Verlaufsform, bei welcher eine primäre intermittierende Form einer sekundären progressiven Verlaufsform voran geht. Bei der zweiten Form der MS gibt es nur einen progressiven Verlauf (Prinz et al., 2011; Ransohoff, 2012). Therapiemaßnahmen bestehen hauptsächlich aus der Verabreichung von antiinflammatorischen Substanzen wie Interferon-ß, welche zu einem langsameren Krankheitsverlauf führen. Trotz aller Bemühungen, ist die Krankheit bis heute nicht heilbar. Die EAE ist ein Tiermodell der MS und simuliert die progressive Verlaufsform dieser Erkrankung. Die verschiedenen EAE-Formen werden häufig in Nagetieren wie Ratten oder Mäusen ausgelöst (Constantinescu et al., 2011; Ransohoff, 2012), um Störungen der Motorik, inflammatorische Demyelinisierung und Gewichtsverlust zu untersuchen. Es gibt zwei Arten eine EAE zu induzieren. Zum einen besteht die Möglichkeit, gegen die Antigene MBP, PLP oder MOG voraktivierte T-Zellen intravenös zu injizieren oder direkt Peptide von 13 MBP, PLP oder MOG in Kombination mit dem vollständigen Freudschen Adjuvans (CFA), einer Emulsion welche das abgetötete Mycobacterium tuberculosis enthält. Durch die Bindung des CFA von den Toll-Like-Rezeptoren kommt es zu einer Immunreaktion gegen das gespritzte, körpereigene Antigen. Ungefähr ab dem 10. Tag zeigen sich die ersten klinischen Symptome, welche sich in einer aszendierenden Lähmung äußern. 1.3 Myeloide Zellen des ZNS Eine wesentliche Komponente des Immunsystems im ZNS sind myeloide Zellen, welche eine besondere Rolle für das Immunprivileg im Gehirn einnehmen, aber auch in der Pathogenese verschiedenster neuroimmunologischer Erkrankungen (Prinz et al., 2011). Alle myeloiden Zellen werden im mononukleären phagozytierenden System (Abbildung 3) zusammengefasst. Abbildung 3: Überblick über (Ransohoff und Cardona, 2010) das mononukleäre phagozytierende System Eine der wichtigsten myeloiden Zellpopulationen des Gehirns ist die Mikroglia, deren Vorläuferzellen zum größten Teil während der Embryogenese vom Dottersack in das Gehirnparenchym migrieren (Ginhoux et al., 2010). Weitere myeloide Zellen im Gehirn sind perivaskuläre, meningeale sowie aus dem Plexus choroideus stammende Makrophagen und DCs (Bulloch et al. 2008, Greter et al. 2005, Prodinger et al. 2011, Ransohoff and Cardona 2010). Diese Zellen stammen von einer gemeinsamen hämatopoetischen Stammzelle (HSC) 14 aus dem Knochenmark ab. Daraus entwickelt sich ein gemeinsamer myeloider Vorläufer, der Common myeloid progenitor (CMP). Aus dem CMP entsteht ein granulozytärer und monozytärer Vorläufer, der Granulocyte monocyte progenitor (GMP), um sich anschließend über den Monocyte dendritic cell progenitor (MDP) in Makrophagen, Monozyten und DCs zu differenzieren (Bulloch et al. 2008, Greter et al. 2005, Prodinger et al. 2011, Ransohoff and Cardona 2010). Unter bestimmten inflammatorischen Bedingungen können Monozyten aus der Peripherie in das Hirnparenchym migrieren, sich der Morphologie der Mikroglia anpassen und deren Aufgaben übernehmen (Bechmann et al., 2005; Varvel et al. 2012). 1.3.1 Dendritische Zellen DCs wurden erstmals im Jahr 1973 beschrieben (Steinman und Cohn, 1973) und erfüllen eine Schlüsselfunktion in der Aktivierung zwischen dem angeborenen und adaptiven Immunsystem. DCs sind spezialisierte antigenprozessierende und -präsentierende Zellen (Satpathy et al., 2012). Generell unterscheidet man zwischen reifen und unreifen DCs. Unter inflammatorischen Bedingungen kommt es durch verschiedene Stimuli wie zum Beispiel durch inflammatorische Zytokine zur Reifung von DCs. Parallel kommt es zur Aufnahme von Antigenen und Migration zu den Lymphknoten bzw. lymphatischen Organen. Durch die Erhöhung der Expression der antigenpräsentierenden Moleküle MHC-I und II sowie der T-Zell-bindenden und co-stimulatorischen Moleküle CD80 und CD86 auf der Zelloberfläche, kommt es zu einer Veränderung des Zellphänotyps. Dabei bezeichnet man die Präsentation der Antigene über die MHC-Moleküle als „Signal 1“ und die Stimulation der T-Zellen über CD80 und CD86 als „Signal 2“. Im lymphatischen Gewebe kommt es dann zur Präsentation der aufgenommenen Antigene, um dort naive T-Zellen zu aktivieren (Steinman et al., 2003a; Steinman et al., 2003b). Auch unter nichtinflammatorischen Bedingungen kommt den „unreifen“ DCs eine wichtige Funktion zu. So sind sie bedeutend für die Vermittlung von Toleranz, indem sie T-Zellen depletieren und für die Proliferation von regulatorischen T-Zellen verantwortlich sind (Mohammad et al., 2012; Steinman et al., 2003b). Da durch sterbende körpereigene Zellen ständig körpereigene Antigene präsentiert werden, ist diese Aufgabe essentiell, um einen Ausbruch von Autoimmunerkrankungen zu vermeiden. Eine wichtige Rolle spielt dabei die periphere Toleranz, welche in der Peripherie außerhalb des lymphatischen Gewebes vermittelt wird, da nicht alle reaktiven T-Zellen durch zentrale Toleranz inaktiviert werden können. Auch unreife DCs können Antigene über die MHC-Moleküle präsentieren. Allerdings kommt es dabei zu einer Veränderung des zweiten Signals, welche eine Unterdrückung einer T-Zell-Antwort, eine T-Zell-Anergie, zur Folge hat (Greenfield et al., 1998). Zusätzlich unterscheidet man zwei Arten von DCs. Die klassischen DCs (cDCs) können überall in Geweben vorkommen (Wu et al., 2009). Weiterhin sind spezielle cDCs in der Haut, 15 Leber und Lunge beschrieben (Mesnil et al. ,2012; Nakamoto et al. 2012; van Rijt et al., 2005). Sie sind überwiegend kurzlebig und werden durch Vorläufer aus dem Blut gebildet. Plasmazytoide DCs (pDCs) besitzen den gleichen Vorläufer wie cDCs, sind aber langlebiger als cDCs und hauptsächlich im Knochenmark und in allen peripheren Organen präsent. Sie sind darauf spezialisiert auf virale Infektionen zu reagieren, indem sie eine massive Interferon-I-Antwort auslösen. Ebenso können sie wie cDCs Antigene präsentieren und eine T-Zellantwort auslösen (Geissmann et al., 2010b). Als Marker für DCs werden in der Maus hauptsächlich CD11c und MHC-II benutzt (Gottfried-Blackmore et al. 2009; Greter et al. 2005; Jung et al. 2002; McMahon et al. 2005; van Rijt et al., 2005). Mit Hilfe einer MHC-IIFärbung konnten DCs in den weichen Hirnhäuten und im Plexus choroideus im Rattenhirn nachgewiesen werden und somit die Theorie über das Fehlen von DCs im ZNS (Sedgwick, 1995) widerlegen (Matyszak und Perry, 1996; McMenamin, 1999). Mit der Entwicklung von transgenen Mauslinien, welche unter dem Promotor von CD11c (Itgax) GFP (Jung et al., 2002) bzw. YFP (Lindquist et al., 2004) exprimieren, konnte auch im gesunden Gehirn die Existenz von parenchymalen CD11c-positiven Zellen nachgewiesen werden (Bulloch et al., 2008; Prodinger et al., 2011). 1.3.2 Makrophagen Makrophagen sind residente und phagozytierende Zellen, die nicht nur im lymphatischen Gewebe, sondern auch in allen anderen Organen und Geweben vorkommen (Geissmann et al., 2010b). Unter nichtinflammatorischen Bedingungen spielen sie eine wichtige Rolle bei der Aufrechterhaltung der Homöostase, indem sie apoptotische Zellen beseitigen und Wachstumsfaktoren sezernieren (Geissmann et al., 2010b; Satpathy et al., 2012). Darüber hinaus besitzen Makrophagen eine Vielfalt an Rezeptoren, um Pathogene schnell zu erkennen, diese zu phagozytieren und mit Hilfe der Produktion von bestimmten inflammatorischen Zytokinen und Chemokinen eine gezielte Immunantwort auszulösen. Als typische Erkennungsmarker für Makrophagen in der Maus werden häufig F4/80 und CD11b verwendet (McMahon et al., 2005). Wie bereits erwähnt, existieren auch im Gehirn Makrophagen. Hier unterscheidet man zwischen den aus der Peripherie abstammenden und hauptsächlich in den Meningen, dem Plexus choroideus oder perivaskulär lokalisierten Makrophagen (Ransohoff und Cardona, 2010) sowie den residenten, parenchymal lokalisierten Makrophagen, der Mikroglia (Ransohoff und Cardona, 2010). 1.3.3 Mikroglia Mikroglia sind die residenten Gewebsmakrophagen des Gehirns. CX3CR1 ist ein typischer Marker für residente Makrophagen. Dieser Marker wird auch genutzt, um Mikroglia zu identifizieren und zu charakterisieren. Weitere häufig verwendete Marker für Mikroglia sind 16 F4/80, CD11b und IBA-1 (Carson et al., 1998; Sedgwick et al., 1991). Auch durch ihre spezifische Expression von CD45 kann man Mikroglia von anderen peripheren Makrophagen und mononukleären Zellen unterscheiden. So exprimieren Mikroglia CD45 auf mittlerem (CD45int) Niveau, während andere Leukozyten CD45 auf hohem (CD45high) Niveau exprimieren (Sedgwick et al., 1991). Unter der Verwendung des Modells der entorhinalen Kortexläsion, welches zu einer anterograden, axonalen Degeneration führt, konnte gezeigt werden, dass Vorläuferzellen der Mikroglia von zirkulierenden, knochenmarksabstammenden Monozyten des Blutes in das adulte Gehirn migrieren können (Bechmann et al., 2005). Später konnte gezeigt werden, dass die von Blutmonozyten abstammenden Mikrogliavorläufer unter bestimmten Voraussetzungen, wie zum Beispiel Bestrahlung, in das adulte Gehirn migrieren (Mildner et al., 2007). Ein Großteil der residenten Mikroglia stammt von einem Mirkogliavorläufer ab, welcher bereits während der Embryogenese aus dem extraembryonalen Dottersack ins Gehirn wandert (Ginhoux et al., 2010; Kierdorf et al., 2013). Damit nehmen residente Mikroglia eine besondere Stellung im mononukleären phagozytierenden System ein. Im gesunden Gehirn und unter nichtinflammatorischen Bedingungen besitzen Mikroglia einen kleinen Zellkörper und lange, ramifizierte Fortsätze. Mit diesen Fortsätzen können sie ihre Umgebung überwachen (Nimmerjahn, 2005). Nach Gewebeschaden bzw. einer Entzündung wechseln Mikroglia ihre Morphologie in einen aktiven amöboiden Zustand. Je nach Art der Aktivierung können Mikroglia akut immunologisch auf die Entzündung reagieren, in dem sie aktiv an einer T-Zellantwort beteiligt sind oder eine schnelle Heilung und Reparatur initiieren, um das Gewebe vor einem größeren Schaden durch eine akute Immunantwort zu schützen (Murphy et al., 2010; Prinz et al., 2011). Mikroglia sind ebenfalls in der Umgestaltung und Beseitigung von synaptischen Strukturen der Neurone involviert. Zudem unterstützen sie den Turnover von Myelin im ZNS (Fitzner et al., 2011; Goldmann und Prinz, 2013; Tremblay, 2011). Unter inflammatorischen Bedingungen, zum Beispiel im Falle einer MS, kann regelmäßig eine Aktivierung und Proliferation von Mikroglia beobachtet werden. Mikroglia können proinflammatorische Proteine sezernieren, welche zu einer Aktivierung weiterer Mikroglia bzw. eingewanderter Leukozyten führt (Babcock et al., 2003; Goldmann und Prinz, 2013; Semple et al., 2010). So wurde in einem Modell der entorhinalen Kortexläsion in Mäusen gezeigt, dass Mikroglia im Gehirn, neben Astrozyten, der Hauptproduzent von CCL2 sind (Babcock et al., 2003). Wie oben beschrieben, ist CCL2 wichtig für die Migration durch das Gefäßendothel in den perivaskulären Raum postkapillärer Venulen. Dadurch bewirkt es die Rekrutierung sowie Aktivierung peripherer Leukozyten und Mikroglia (Babcock et al., 2003; Mahad und Ransohoff, 2003; Owens et al., 2008; Semple et al., 2010). 17 Bei einer EAE wurde gezeigt, dass auch Mikroglia als antigenpräsentierene Zellen agieren, in dem sie die dafür wichtigen Oberflächenmoleküle wie MHC-II, CD80 und CD86 exprimieren. Durch die Aufnahme und Prozessierung von Myelin zu kleinen Peptiden, welche dann über MHC-II den myelinspezifischen, autoreaktiven T-Helfer-Zellen präsentiert werden, kann es zum „Epitope spreading“ kommen (Goldmann und Prinz, 2013; Mack et al., 2003; McMahon et al., 2005; Satoh et al., 1995; Tran et al., 1998). Dabei werden diese autoreaktiven T-Zellen aktiviert, welche dann wiederum über die Expression proinflammatorischer Zytokine zu einer weiteren Infiltration peripherer Leukozyten bzw. zur Aktivierung weiterer peripherer Makrophagen und Mikroglia führt (Constantinescu et al., 2011; Goldmann und Prinz, 2013; Mack et al., 2003; McMahon et al., 2005; Ortiz et al., 2014). Andererseits konnte beobachtet werden, dass Mikroglia auch Toleranz induzieren können, indem sie das 2. Signal der Antigenpräsentation so veränderten, dass es zu einer Anergie der infiltrierten antigenspezifischen T-Zellen kam (Bechmann et al., 2001b; Kuchroo et al., 1995). 1.4 CD11c als Marker für Dendritische Zellen Einer der am häufigsten verwendeten Marker für verschiedene Subpopulationen der DCs ist CD11c. Da jedoch gezeigt werden konnte, dass dieser Marker nicht nur allein auf DCs exprimiert wird, sondern auch auf anderen Zelltypen, wie den Makrophagen, wird die Existenz von DCs als eigenständige Zellpopulation in Frage gestellt (Geissmann et al., 2010a; Hume, 2008; Randolph und Merad, 2013). Dabei scheint, dass die Berücksichtigung der Herkunft dieser Zellen eine wichtige Rolle spielt. So sollte beachtet werden, ob man Zellen aus einem lymphatischen Gewebe oder nichtlymphatischen Gewebe beschreibt. Typische Charakterisierungsmarker von Zellen des mononukleären Systems wie MHC-II, F4/80, CD11b und CD11c, sind nicht unbedingt vergleichbar zwischen den einzelnen Populationen und Geweben (Geissmann et al., 2010a; Merad et al., 2013). Prinzipiell unterscheidet man DCs durch ihre Expression von CD11c und MHC-II von Makrophagen. Makrophagen zeichnen sich eher durch ihre Expression von F4/80 und CD11b aus (Bulloch et al., 2008; Ji et al., 2013; Jung et al., 2002; McMahon et al., 2005). Jedoch gibt es Arbeiten, welche zeigten, dass auch Lungen- und Milzmakrophagen fähig sind, CD11c zu exprimieren (Probst et al., 2005; van Rijt et al., 2005). Darüber hinaus wurde beschrieben, dass auch DCs typische Makrophagenmarker aufweisen können (Bogunovic et al., 2009; Denning et al., 2007; Merad et al., 2013). Zum Beispiel wurden F4/80-positive Zellen der Lamina propria aufgrund der Expression von CD11c als DCs beschrieben (Bogunovic et al., 2009). In einer anderen Arbeit wurden die gleichen Zellen als Makrophagenpopulation bezeichnet. Obwohl die Autoren diese Zellen hier als Makrophagen bezeichnen, wurde gezeigt, dass diese Population regulatorische T-Zellen induzieren kann und wichtig für die Vermittlung der 18 Toleranz in der Lamina propria des Darms ist, eine Eigenschaft die vornehmlich DCs zugesprochen wird (Denning et al., 2007). Weiterhin konnte im Gehirn unter inflammatorischen Bedingungen gezeigt werden, dass auch eingewanderte Makrophagen den DC-Marker CD11c exprimieren (McMahon et al., 2005). Ein weiteres funktionelles Merkmal für die Charakterisierung von DCs ist die Antigenpräsentation gegenüber naiven TZellen. Auch hier konnte in verschiedenen Tiermodellen beobachtet werden, dass Makrophagen fähig sind, Antigene naiven T-Zellen zu präsentieren (Mesnil et al., 2012; Nakamoto et al., 2012). Demzufolge ergibt sich daraus das Problem, dass keine uniforme Definition von DCs bzw. Makrophagen in den untersuchten Geweben, als auch unter den verschiedenen Voraussetzungen, unter welchen diese Charakterisierungen stattfanden, existiert (Geissmann et al., 2010a; Hume, 2008; Hume et al., 2013; Randolph und Merad, 2013). Durch die Verwendung des transgenen Tiermodells CD11c-DTR/GFP (Jung et al., 2002) zeigten wir erstmals intraparenchymal lokalisierte CD11c-positive Zellen unter gesunden Bedingungen im Gehirn. Wir konnten zeigen, dass diese Zellen vorwiegend positiv für die Mikroglia/Makrophagenmarker Iba-1 und CD11b sind. Bis jetzt existiert keine einheitliche Definition dieser intraparenchymal lokalisierten Zellen unter nichtinflammatorischen Voraussetzungen. Aufgrund der oben beschriebenen Heterogenität in der Charakterisierung von Makrophagen und DCs sowie mit dem Aufkommen der Diskussion, ob DCs als unabhängige und klar definierte Zelllinie überhaupt existieren (Geissmann et al., 2010a; Hume, 2008; Hume et al., 2013; Randolph und Merad, 2013), stellte sich uns die Frage, wie sich intraparenchymale CD11c-positive Zellen des Gehirns von CD11c-positiven Zellen aus anderen Organen unterscheiden. Dafür verglichen wir diese auf die Expression spezifischer monozytärer und inflammatorischer Zellmarker. 19 2 Fragestellung Durch die Heterogenität in der Charakterisierung von DCs ist die Existenz derselben als eigenständige Zelllinie umstritten, da bislang keine klare Definition existiert. Zudem konnte gezeigt werden, dass einer der Hauptmarker für DCs, CD11c, auch auf anderen Zelltypen wie Makrophagen exprimiert werden kann. Ziel dieser Arbeit war daher, die von uns identifizierten CD11c-positiven Zellen mit CD11c-positiven Zellen aus Lunge, Leber und Milz immunphänotypisch zu vergleichen. Dadurch wollten wir Klarheit erlangen inwiefern Ähnlichkeiten zwischen den untersuchten Zellen den Begriff Dendritische Zelle reflektieren. Dabei untersuchten wir die folgenden Fragestellungen: 1. Wie unterscheiden sich CD11c-positive Zellen im Gehirn von CD11c-positiven Zellen aus anderen Organen? 2. Existieren CD11c-positive Mikroglia als eigenständige Population? 3. Besitzen intraparenchymale CD11c-positive Zellen intrinsische Eigenschaften oder können diese abhängig vom Umgebungsmillieu beeinflusst werden? Im Rahmen dieser angefertigten Arbeit konnten wir zeigen, dass die intraparenchymale CD11c-positive Population im Gehirn immunphänotypisch den Mikroglia ähnelt und sich vor allem in ihrer MHC-II-Expression von den anderen untersuchten CD11c-positiven Populationen unterscheidet. Auch die MHC-II-Expression unterscheidet sich zwischen den CD11c-positiven Mikroglia und den CD11c-negativen Mikroglia nicht. Bei der Untersuchung, ob sich die MHC-II-Expression der Mikroglia in der Umgebung eines Immunorgans ändern kann, beobachteten wir in unseren Experimenten keine Veränderung der MHC-II-Expression und schlussfolgern daher, dass Mikroglia einen intrinsischen Phänotyp besitzen. Dieser einzigartige Phänotyp der CD11c-positiven Mikroglia könnte einen Einfluss auf das Immunprivileg des Gehirns vermuten lassen. Zudem erlaubt die Charakterisierung der CD11c-positiven Zellen im Gehirn und deren immunphänotypischer Vergleich mit peripheren CD11c-positiven Populationen die Untersuchung von Veränderungen im Rahmen der Neuroinflammation und könnte so zu einem besseren Verständnis der Pathophysiologie zahlreicher Erkrankungen wie der MS beitragen. 20 3 Literaturverzeichnis der Einleitung Agrawal, S.; Anderson, P.; Durbeej, M.; van Rooijen, N.; Ivars, F.; Opdenakker, G.; Sorokin, L. M. (2006): Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis, Journal of Experimental Medicine (Band 203), Nr. 4, Seite 10071019. Ajami, B.; Bennett, J. L.; Krieger, C.; McNagny, K. M.; Rossi, F. M. (2011): Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool, Nature Neuroscience (Band 14), Nr. 9, Seite 1142-1149. Andersson, P. B.; Perry, V. H.; Gordon, S. (1992): The acute inflammatory response to lipopolysaccharide in CNS parenchyma differs from that in other body tissues, Neuroscience (Band 48), Nr. 1, Seite 169-186. Andres, K.; von Düring, M.; Muszynski, K.; Schmidt, R. (1987): Nerve fibres and their terminals of the dura mater encephali of the rat., Anatomy and Embryology (Band 175), Nr. 3, Seite 289-301. Angelov, D. N.; Walther, M.; Streppel, M.; Guntinas-Lichius, O.; Neiss, W. F. (1998): The cerebral perivascular cells, Advance in Anatomy Embryology and Cell Biology (Band 147), Seite 1-87. Babcock, A. A.; Kuziel, W. A.; Rivest, S.; Owens, T. (2003): Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS, Journal of Neuroscience (Band 23), Nr. 21, Seite 7922-7930. Barker, C. F.; Billingham, R. E. (1977): Immunologically privileged sites, Advances in Immunology (Band 25), Seite 1-54. Bechmann, I.; Galea, I.; Perry, V. H. (2007): What is the blood-brain barrier (not)?, Trends in Immunology (Band 28), Nr. 1, Seite 5-11. Bechmann, I.; Goldmann, J.; Kovac, A. D.; Kwidzinski, E.; Simburger, E.; Naftolin, F.; Dirnagl, U.; Nitsch, R.; Priller, J. (2005): Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia, Journal of Federation of American Societies for Experimental Biology (Band 19), Nr. 6, Seite 647-649. Bechmann, I.; Kwidzinski, E.; Kovac, A. D.; Simburger, E.; Horvath, T.; Gimsa, U.; Dirnagl, U.; Priller, J.; Nitsch, R. (2001a): Turnover of rat brain perivascular cells, Experimental Neurology (Band 168), Nr. 2, Seite 242-249. Bechmann, I.; Mor, G.; Nilsen, J.; Eliza, M.; Nitsch, R.; Naftolin, F. (1999): FasL (CD95L, Apo1L) is expressed in the normal rat and human brain: evidence for the existence of an immunological brain barrier, Glia (Band 27), Nr. 1, Seite 62-74. 21 Bechmann, I.; Peter, S.; Beyer, M.; Gimsa, U.; Nitsch, R. (2001b): Presence of B7--2 (CD86) and lack of B7--1 (CD(80) on myelin phagocytosing MHC-II-positive rat microglia is associated with nondestructive immunity in vivo, FASEB journal : official publication of Journal of Federation of American Societies for Experimental Biology (Band 15), Nr. 6, Seite 1086–1088. Billingham, R. E.; Boswell, T. (1953): Studies on the problem of corneal homografts, Proceedings of the Royal Society of London B Biological Science (Band 141), Nr. 904, Seite 392-406. Bogunovic, M.; Ginhoux, F.; Helft, J.; Shang, L.; Hashimoto, D.; Greter, M.; Liu, K.; Jakubzick, C.; Ingersoll, M. A.; Leboeuf, M.; Stanley, E. R.; Nussenzweig, M.; Lira, S. A.; Randolph, G. J.; Merad, M. (2009): Origin of the lamina propria dendritic cell network, Immunity (Band 31), Nr. 3, Seite 513-525. Bulloch, K.; Miller, M. M.; Gal-Toth, J.; Milner, T. A.; Gottfried-Blackmore, A.; Waters, E. M.; Kaunzner, U. W.; Liu, K.; Lindquist, R.; Nussenzweig, M. C.; Steinman, R. M.; McEwen, B. S. (2008): CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain, Journal of Comparative Neurology (Band 508), Nr. 5, Seite 687-710. Carson, M. J.; Reilly, C. R.; Sutcliffe, J. G.; Lo, D. (1998): Mature microglia resemble immature antigen-presenting cells, Glia (Band 22), Nr. 1, Seite 72–85. Constantinescu, C. S.; Farooqi, N.; O'Brien, K.; Gran, B. (2011): Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS), British Journal of Pharmacology (Band 164), Nr. 4, Seite 1079-1106. Correale, J.; Villa, A. (2007): The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting, Autoimmunity (Band 40), Nr. 2, Seite 148-160. Cserr, H. F.; Knopf, P. M. (1992): Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view, Immunology Today (Band 13), Nr. 12, Seite 507-512. Denning, T. L.; Wang, Y. C.; Patel, S. R.; Williams, I. R.; Pulendran, B. (2007): Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses, Nature Immunology (Band 8), Nr. 10, Seite 1086-1094. Ehrlich, P. (1885): Das Sauerstoff-Bedürfnis des Organismus. Eine farbenanalytische Studie, Verlag von August Hirschwald, Berlin. Engelhardt, B.; Ransohoff, R. M. (2005): The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms, Trends in Immunology (Band 26), Nr. 9, Seite 485-495. 22 Fabriek, B. O.; Van Haastert, E. S.; Galea, I.; Polfliet, M. M.; Dopp, E. D.; Van Den Heuvel, M. M.; Van Den Berg, T. K.; De Groot, C. J.; Van Der Valk, P.; Dijkstra, C. D. (2005): CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation, Glia (Band 51), Nr. 4, Seite 297-305. Finsen, B. R.; Sorensen, T.; Castellano, B.; Pedersen, E. B.; Zimmer, J. (1991): Leukocyte infiltration and glial reactions in xenografts of mouse brain tissue undergoing rejection in the adult rat brain. A light and electron microscopical immunocytochemical study, Journal of Neuroimmunology (Band 32), Nr. 2, Seite 159-183. Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U. K.; Simons, M. (2011): Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis, Journal of Cell Science (Band 124), Nr. Pt 3, Seite 447-458. Galea, I.; Bechmann, I.; Perry, V. H. (2007): What is immune privilege (not)?, Trends in Immunology (Band 28), Nr. 1, Seite 12–18. Geissmann, F.; Gordon, S.; Hume, D. A.; Mowat, A. M.; Randolph, G. J. (2010a): Unravelling mononuclear phagocyte heterogeneity, Nature Reviews Immunology (Band 10), Nr. 6, Seite 453–460. Geissmann, F.; Manz, M. G.; Jung, S.; Sieweke, M. H.; Merad, M.; Ley, K. (2010b): Development of Monocytes, Macrophages, and Dendritic Cells, Science (Band 327), Nr. 5966, Seite 656–661. Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M. F.; Conway, S. J.; Ng, L. G.; Stanley, E. R.; Samokhvalov, I. M.; Merad, M. (2010): Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages, Science (Band 330), Nr. 6005, Seite 841–845. Goldmann, J.; Kwidzinski, E.; Brandt, C.; Mahlo, J.; Richter, D.; Bechmann, I. (2006): T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa, Journal of Leukocyte Biology (Band 80), Nr. 4, Seite 797–801. Goldmann, T.; Prinz, M. (2013): Role of microglia in CNS autoimmunity, Clinical & Developmental Immunology (Band 2013), Seite 208093. Greenfield, E. A.; Nguyen, K. A.; Kuchroo, V. K. (1998): CD28/B7 costimulation: a review, Critical Reviews in Immunology (Band 18), Nr. 5, Seite 389-418. Greter, M.; Heppner, F. L.; Lemos, M. P.; Odermatt, B. M.; Goebels, N.; Laufer, T.; Noelle, R. J.; Becher, B. (2005): Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis, Nature Medicine (Band 11), Nr. 3, Seite 328–334. Hickey, W. F. (2001): Basic principles of immunological surveillance of the normal central nervous system, Glia (Band 36), Nr. 2, Seite 118-124. 23 Hickey, W. F.; Kimura, H. (1988): Perivascular microglial cells of the CNS are bone marrowderived and present antigen in vivo, Science (Band 239), Nr. 4837, Seite 290-292. Huang, D.; Tani, M.; Wang, J.; Han, Y.; He, T. T.; Weaver, J.; Charo, I. F.; Tuohy, V. K.; Rollins, B. J.; Ransohoff, R. M. (2002): Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice, Journal of Neuroscience (Band 22), Nr. 24, Seite 10633-10642. Hume, D. A. (2008): Macrophages as APC and the dendritic cell myth, Journal of Immunology (Baltimore, Md. : 1950) (Band 181), Nr. 9, Seite 5829–5835. Hume, D. A.; Mabbott, N.; Raza, S.; Freeman, T. C. (2013): Can DCs be distinguished from macrophages by molecular signatures?, Nature Immunology (Band 14), Nr. 3, Seite 187–189. Ichimura, T.; Fraser, P. A.; Cserr, H. F. (1991): Distribution of extracellular tracers in perivascular spaces of the rat brain, Brain Research (Band 545), Nr. 1-2, Seite 103113. Jensen, M. B.; Finsen, B.; Zimmer, J. (1997): Morphological and immunophenotypic microglial changes in the denervated fascia dentata of adult rats: correlation with blood-brain barrier damage and astroglial reactions, Experimental Neurology (Band 143), Nr. 1, Seite 103-116. Ji, Q.; Castelli, L.; Goverman, J. M. (2013): MHC class I–restricted myelin epitopes are crosspresented by Tip-DCs that promote determinant spreading to CD8+ T cells, Nature Immunology (Band 14), Nr. 3, Seite 254–261. Jung, S.; Unutmaz, D.; Wong, P.; Sano, G.-I.; los Santos, K. d.; Sparwasser, T.; Wu, S.; Vuthoori, S.; Ko, K.; Zavala, F.; Pamer, E. G.; Littman, D. R.; Lang, R. A. (2002): In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens, Immunity (Band 17), Nr. 2, Seite 211–220. Kaminski, M.; Bechmann, I.; Kiwit, J.; Glumm, J. (2012): Migration of monocytes after intracerebral injection, Cell Adhesion & Migration (Band 6), Nr. 3, Seite 164–167. Kida, S.; Pantazis, A.; Weller, R. O. (1993): CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance, Neuropathology and Applied Neurobiology (Band 19), Nr. 6, Seite 480-488. Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E. G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; Müller, D. N.; Luckow, B.; Brocker, T.; Debowski, K.; Fritz, G.; Opdenakker, G.; Diefenbach, A.; Biber, K.; Heikenwalder, M.; Geissmann, F.; Rosenbauer, F.; Prinz, M. (2013): Microglia emerge from erythromyeloid precursors via Pu.1- and Neuroscience (Band 16), Nr. 3, Seite 273–280. Irf8-dependent pathways, Nature 24 Krueger, M.; Bechmann, I. (2010): CNS pericytes: Concepts, misconceptions, and a way out, Glia (Band 58), Nr. 1, Seite 1–10. Kuchroo, V. K.; Das, M. P.; Brown, J. A.; Ranger, A. M.; Zamvil, S. S.; Sobel, R. A.; Weiner, H. L.; Nabavi, N.; Glimcher, L. H. (1995): B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy, Cell (Band 80), Nr. 5, Seite 707–718. Lassmann, H.; van Horssen, J.; Mahad, D. (2012): Progressive multiple sclerosis: pathology and pathogenesis, Nature Reviews Neurology (Band 8), Nr. 11, Seite 647–656. Lewandowski, M. (1900): Zur Lehre von der Cerebrospinalflüssigkeit., Zeitschrift für klinische Forschung (Band 40), Seite 14. Lindquist, R. L.; Shakhar, G.; Dudziak, D.; Wardemann, H.; Eisenreich, T.; Dustin, M. L.; Nussenzweig, M. C. (2004): Visualizing dendritic cell networks in vivo, Nature Immunology (Band 5), Nr. 12, Seite 1243–1250. Locatelli, G.; Wörtge, S.; Buch, T.; Ingold, B.; Frommer, F.; Sobottka, B.; Krüger, M.; Karram, K.; Bühlmann, C.; Bechmann, I.; Heppner, F. L.; Waisman, A.; Becher, B. (2012): Primary oligodendrocyte death does not elicit anti-CNS immunity, Nature Neuroscience (Band 15), Nr. 4, Seite 543–550. Mack, C. L.; Vanderlugt-Castaneda, C. L.; Neville, K. L.; Miller, S. D. (2003): Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis, Journal of Neuroimmunology (Band 144), Nr. 1-2, Seite 68-79. Mahad, D. J.; Ransohoff, R. M. (2003): The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE), Seminars Immunology (Band 15), Nr. 1, Seite 23-32. Mason, D. W.; Charlton, H. M.; Jones, A. J.; Lavy, C. B.; Puklavec, M.; Simmonds, S. J. (1986): The fate of allogeneic and xenogeneic neuronal tissue transplanted into the third ventricle of rodents, Neuroscience (Band 19), Nr. 3, Seite 685-694. Matyszak, M. K.; Perry, V. H. (1996): The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system, Neuroscience (Band 74), Nr. 2, Seite 599–608. Matyszak, M. K.; Perry, V. H. (1998): Bacillus Calmette-Guérin sequestered in the brain parenchyma escapes immune recognition, Journal of Neuroimmunology (Band 82), Nr. 1, Seite 73–80. McMahon, E. J.; Bailey, S. L.; Castenada, C. V.; Waldner, H.; Miller, S. D. (2005): Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis, Nature Medicine (Band 11), Nr. 3, Seite 335–339. 25 McMenamin, P. G. (1999): Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations, The Journal of Comparative Neurology (Band 405), Nr. 4, Seite 553–562. McMenamin, P. G.; Wealthall, R. J.; Deverall, M.; Cooper, S. J.; Griffin, B. (2003): Macrophages and dendritic cells in the rat meninges and choroid plexus: threedimensional localisation by environmental scanning electron microscopy and confocal microscopy, Cell and Tissue Research (Band 313), Nr. 3, Seite 259–269. Medawar, P. B. (1948): Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye, British Journal of Experimental Pathology (Band 29), Nr. 1, Seite 58-69. Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. (2013): The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting, Annual Review of Immunology (Band 31), Seite 563-604. Mesnil, C.; Sabatel, C. M.; Marichal, T.; Toussaint, M.; Cataldo, D.; Drion, P.-V.; Lekeux, P.; Bureau, F.; Desmet, C. J.; Ryffel, B. (2012): Resident CD11b+Ly6C− Lung Dendritic Cells Are Responsible for Allergic Airway Sensitization to House Dust Mite in Mice, PLoS ONE (Band 7), Nr. 12, Seite e53242. Mildner, A.; Schmidt, H.; Nitsche, M.; Merkler, D.; Hanisch, U. K.; Mack, M.; Heikenwalder, M.; Bruck, W.; Priller, J.; Prinz, M. (2007): Microglia in the adult brain arise from Ly6ChiCCR2+ monocytes only under defined host conditions, Nature Neuroscience (Band 10), Nr. 12, Seite 1544-1553. Mohammad, M. G.; Hassanpour, M.; Tsai, V. W. W.; Li, H.; Ruitenberg, M. J.; Booth, D. W.; Serrats, J.; Hart, P. H.; Symonds, G. P.; Sawchenko, P. E.; Breit, S. N.; Brown, D. A. (2012): Dendritic cells and multiple sclerosis: disease, tolerance and therapy, International Journal of Molecular Sciences (Band 14), Nr. 1, Seite 547–562. Muller, D. M.; Pender, M. P.; Greer, J. M. (2005): Blood-brain barrier disruption and lesion localisation in experimental autoimmune encephalomyelitis with predominant cerebellar and brainstem involvement, Journal of Neuroimmunology (Band 160), Nr. 1-2, Seite 162-169. Murphy, Á. C.; Lalor, S. J.; Lynch, M. A.; Mills, K. H. G. (2010): Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis, Brain, Behavior, and Immunity (Band 24), Nr. 4, Seite 641–651. Murphy, J. B.; Sturm, E. (1923): Conditions determining the transplantability of tissues in the brain, The Journal of Experimental Medicine (Band 38), Nr. 2, Seite 183–197. 26 Mutlu, L.; Brandt, C.; Kwidzinski, E.; Sawitzki, B.; Gimsa, U.; Mahlo, J.; Aktas, O.; Nitsch, R.; Zwam, M.; Laman, J. D.; Bechmann, I. (2007): Tolerogenic effect of fiber tract injury: reduced EAE severity following entorhinal cortex lesion, Experimental Brain Research (Band 178), Nr. 4, Seite 542–553. Nakamoto, N.; Ebinuma, H.; Kanai, T.; Chu, P. S.; Ono, Y.; Mikami, Y.; Ojiro, K.; Lipp, M.; Love, P. E.; Saito, H.; Hibi, T. (2012): CCR9+ macrophages are required for acute liver inflammation in mouse models of hepatitis, Gastroenterology (Band 142), Nr. 2, Seite 366-376. Nimmerjahn, A. (2005): Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo, Science (Band 308), Nr. 5726, Seite 1314–1318. Ortiz, G. G.; Pacheco-Moises, F. P.; Macias-Islas, M. A.; Flores-Alvarado, L. J.; MirelesRamirez, M. A.; Gonzalez-Renovato, E. D.; Hernandez-Navarro, V. E.; SanchezLopez, A. L.; Alatorre-Jimenez, M. A. (2014): Role of the Blood-Brain Barrier in Multiple Sclerosis, Archives of Medical Research (Band 45), Nr. 8, Seite 687-697. Owens, T.; Bechmann, I.; Engelhardt, B. (2008): Perivascular spaces and the two steps to neuroinflammation, Journal of Neuropathology & Experimental Neurology (Band 67), Nr. 12, Seite 1113-1121. Perry, V. H. (1998): A revised view of the central nervous system microenvironment and major histocompatibility complex class II antigen presentation, Journal of Neuroimmunology (Band 90), Nr. 2, Seite 113-121. Priller, J.; Flugel, A.; Wehner, T.; Boentert, M.; Haas, C. A.; Prinz, M.; Fernandez-Klett, F.; Prass, K.; Bechmann, I.; de Boer, B. A.; Frotscher, M.; Kreutzberg, G. W.; Persons, D. A.; Dirnagl, U. (2001): Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment, Nature Medicine (Band 7), Nr. 12, Seite 1356-1361. Prinz, M.; Priller, J.; Sisodia, S. S.; Ransohoff, R. M. (2011): Heterogeneity of CNS myeloid cells and their roles in neurodegeneration, Nature Neuroscience (Band 13), Nr. 10, Seite 1227–1235. Raine, C. S.; Cannella, B.; Duijvestijn, A. M.; Cross, A. H. (1990): Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination, Laboratory Investigation (Band 63), Nr. 4, Seite 476-489. Randolph, G.; Merad, M. (2013): Reply to: "Can DCs be distinguished from macrophages by molecular signatures?", Nature Immunology (Band 14), Nr. 3, Seite 189–190. Ransohoff, R. M. (2012): Animal models of multiple sclerosis: the good, the bad and the bottom line, Nature Neuroscience (Band 15), Nr. 8, Seite 1074–1077. 27 Ransohoff, R. M.; Cardona, A. E. (2010): The myeloid cells of the central nervous system parenchyma, Nature (Band 468), Nr. 7321, Seite 253-262. Reese, T. S.; Karnovsky, M. J. (1967): Fine structural localization of a blood-brain barrier to exogenous peroxidase, The Journal of Cell Biology (Band 34), Nr. 1, Seite 207-217. Sabelko-Downes, K. A.; Cross, A. H.; Russell, J. H. (1999): Dual role for Fas ligand in the initiation of and recovery from experimental allergic encephalomyelitis, Journal of Experimental Medicine (Band 189), Nr. 8, Seite 1195-1205. Satoh, J.; Lee, Y. B.; Kim, S. U. (1995): T-cell costimulatory molecules B7-1 (CD80) and B72 (CD86) are expressed in human microglia but not in astrocytes in culture, Brain Research (Band 704), Nr. 1, Seite 92-96. Satpathy, A. T.; Wu, X.; Albring, J. C.; Murphy, K. M. (2012): Re(de)fining the dendritic cell lineage, Nature Immunology (Band 13), Nr. 12, Seite 1145–1154. Sedgwick, J. D. (1995): Immune surveillance and autoantigen recognition in the central nervous system, Australian & New Zealand Journal of Medicine (Band 25), Nr. 6, Seite 784-792. Sedgwick, J. D.; Schwender, S.; Imrich, H.; Dörries, R.; Butcher, G. W.; ter Meulen, V. (1991): Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system, Proceedings of the National Academy of Sciences of the United States of America (Band 88), Nr. 16, Seite 7438–7442. Semple, B. D.; Kossmann, T.; Morganti-Kossmann, M. C. (2010): Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks, Journal of Cerebral Blood Flow Metabolism (Band 30), Nr. 3, Seite 459473. Shirai, Y. (1921): On the transplantation of the rat sarcoma in adult heterogenous animals, Japanese Medical World (Band 1), Seite 14-15. Sixt, M.; Engelhardt, B.; Pausch, F.; Hallmann, R.; Wendler, O.; Sorokin, L. M. (2001): Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis, Journal of Cell Biology (Band 153), Nr. 5, Seite 933-946. Steinman, L. (1996): Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system, Cell (Band 85), Nr. 3, Seite 299-302. Steinman, R. M.; Cohn, Z. A. (1973): Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution, Journal of Experimental Medicine (Band 137), Nr. 5, Seite 1142-1162. Steinman, R. M.; Hawiger, D.; Liu, K.; Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Iyoda, T.; Ravetch, J.; Dhodapkar, M.; Inaba, K.; Nussenzweig, M. (2003a): Dendritic cell 28 function in vivo during the steady state: a role in peripheral tolerance, Annals of the New York Academy of Sciences (Band 987), Seite 15–25. Steinman, R. M.; Hawiger, D.; Nussenzweig, M. C. (2003b): T OLEROGENIC D ENDRITIC C ELLS *, Annual Review of Immunology (Band 21), Nr. 1, Seite 685–711. Stevenson, P. G.; Hawke, S.; Sloan, D. J.; Bangham, C. R. (1997): The immunogenicity of intracerebral virus infection depends on anatomical site, Journal of Virology (Band 71), Nr. 1, Seite 145-151. Toft-Hansen, H.; Buist, R.; Sun, X. J.; Schellenberg, A.; Peeling, J.; Owens, T. (2006): Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system, Journal of Immunology (Band 177), Nr. 10, Seite 7242-7249. Tran, E. H.; Hoekstra, K.; van Rooijen, N.; Dijkstra, C. D.; Owens, T. (1998): Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice, Journal of Immunology (Band 161), Nr. 7, Seite 37673775. Tremblay, M. E. (2011): The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies, Neuron Glia Biology (Band 7), Nr. 1, Seite 67-76. van Zwam, M.; Huizinga, R.; Melief, M.-J.; Wierenga-Wolf, A. F.; Meurs, M.; Voerman, J. S.; Biber, K. P. H.; Boddeke, H. W. G. M.; Höpken, U. E.; Meisel, C.; Meisel, A.; Bechmann, I.; Hintzen, R. Q.; ‘t Hart, B. A.; Amor, S.; Laman, J. D.; Boven, L. A. (2009): Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE, Journal of Molecular Medicine (Band 87), Nr. 3, Seite 273–286. Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P. O. (2009): The blood-brain barrier in brain homeostasis and neurological diseases, Biochimica et Biophysica Acta (Band 1788), Nr. 4, Seite 842-857. Wekerle, H. (1993): Experimental autoimmune encephalomyelitis as a model of immunemediated CNS disease, Current Opinion in Neurobiology (Band 3), Nr. 5, Seite 779784. Wolburg, H.; Wolburg-Buchholz, K.; Engelhardt, B. (2005): Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact, Acta Neuropathologica (Band 109), Nr. 2, Seite 181-190. Wu, C.; Ivars, F.; Anderson, P.; Hallmann, R.; Vestweber, D.; Nilsson, P.; Robenek, H.; Tryggvason, K.; Song, J.; Korpos, E.; Loser, K.; Beissert, S.; Georges-Labouesse, E.; Sorokin, L. M. (2009): Endothelial basement membrane laminin α5 selectively inhibits 29 T lymphocyte extravasation into the brain, Nature Medicine (Band 15), Nr. 5, Seite 519–527. 30 4 Publikationen 4.1 CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system Carolin Prodinger, Jörg Bunse, Martin Krüger, Fridtjof Schiefenhövel, Christine Brandt, Jon D. Laman, Melanie Greter, Kerstin Immig, Frank Heppner, Burkhard Becher, Ingo Bechmann Carolin Prodinger, Jörg Bunse have contributed equally. C. Prodinger , M. Krüger, F. Schiefenhövel, I. Bechmann Institute of Clinical Neuroanatomy, Johann Wolfgang Goethe-University, 60590 Frankfurt/Main, Germany e-mail: [email protected] J. Bunse, M. Krüger, F. Schiefenhövel, K. Immig, I. Bechmann Institute of Anatomy, University of Leipzig, 04103 Leipzig, Germany J. Bunse, C. Brandt Institute of Cell and Neurobiology, Charité,10098 Berlin, Germany J. D. Laman Department of Immunology, MS Centre ErasMS, Erasmus MC, 3000 DR Rotterdam, The Netherlands M. Greter, B. Becher Neuroimmunology Unit, Institute of Experimental Immunology, University Hospital of Zurich, Zurich, Switzerland F. Heppner Institute for Neuropathology, Charité, 10098 Berlin, Germany 31 Acta Neuropathol (2011) 121:445–458 DOI 10.1007/s00401-010-0774-y ORIGINAL PAPER CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system Carolin Prodinger • Jörg Bunse • Martin Krüger • Fridtjof Schiefenhövel Christine Brandt • Jon D. Laman • Melanie Greter • Kerstin Immig • Frank Heppner • Burkhard Becher • Ingo Bechmann • Received: 9 August 2010 / Revised: 27 October 2010 / Accepted: 31 October 2010 / Published online: 13 November 2010 Ó Springer-Verlag 2010 Abstract Recent studies demonstrated that primary immune responses can be induced within the brain depending on vessel-associated cells expressing markers of dendritic cells (DC). Using mice transcribing the green fluorescent protein (GFP) under the promoter of the DC marker CD11c, we determined the distribution, phenotype, and source of CD11c? cells in non-diseased brains. Predilection areas of multiple sclerosis (MS) lesions (periventricular area, adjacent fibre tracts, and optical nerve) were preferentially populated by CD11c? cells. Most CD11c? cells were located within the juxtavascular parenchyma rather than the perivascular spaces. Virtually Carolin Prodinger, Jörg Bunse have contributed equally. C. Prodinger M. Krüger F. Schiefenhövel I. Bechmann (&) Institute of Clinical Neuroanatomy, Johann Wolfgang Goethe-University, 60590 Frankfurt/Main, Germany e-mail: [email protected] J. Bunse M. Krüger F. Schiefenhövel K. Immig I. Bechmann Institute of Anatomy, University of Leipzig, 04103 Leipzig, Germany J. Bunse C. Brandt Institute of Cell and Neurobiology, Charité, 10098 Berlin, Germany all CD11c? cells co-expressed ionized calcium-binding adaptor molecule 1 (IBA-1), CD11b, while detectable levels of major histocompatibility complex II (MHC-II) in non-diseased mice was restricted to CD11c? cells of the choroid plexus. Cellular processes project into the glia limitans which may allow transport and/or presentation of intraparenchymal antigens to extravasated T cells in perivascular spaces. In chimeric mice bearing CD11c-GFP bone marrow, fluorescent cells appeared in the CNS between 8 and 12 weeks after transplantation. In organotypic slice cultures from CD11c-GFP mice, the number of fluorescent cells strongly increased within 72 h. Strikingly, using anti-CD209, an established marker for human DC, a similar population was detected in human brains. Thus, we show for the first time that CD11c? cells can not only be recruited from the blood into the parenchyma, but also develop from an intraneural precursor in situ. Dysbalance in their recruitment/development may be an initial step in the pathogenesis of chronic (autoimmune) neuroinflammatory diseases such as MS. Keywords Neurodegeneration Alzheimer Innate immunity Multiple sclerosis (MS) Microglia Immune privilege Introduction J. D. Laman Department of Immunology, MS Centre ErasMS, Erasmus MC, 3000 DR Rotterdam, The Netherlands M. Greter B. Becher Neuroimmunology Unit, Institute of Experimental Immunology, University Hospital of Zurich, Zurich, Switzerland F. Heppner Institute for Neuropathology, Charité, Berlin, Germany Based on the observation that many foreign antigens, such as allografts or heat-killed Bacillus Calmette-Guérin (BCG), inoculated into the parenchyma are tolerated [31, 35, 43] the brain has been addressed as an ‘‘immunologically privileged’’ site [4, 8, 14]. The concept of immune privilege has often been misapprehended as describing a state of ignorance, although Medawar had already shown 123 32 that the observed tolerance to ‘‘foreign’’ antigens within the brain parenchyma can readily be broken by peripherally exposing the same antigen [3, 35]. Therefore, he concluded ‘‘that skin homografts transplanted to the brain submit to, but cannot elicit an immune state’’ and that ‘‘a lymphatic drainage system is necessary for immunity to be called into being’’ [35]. Such drainage is now established for solutes involving pathways such as perivascular spaces, the cribroid plate, and the perineural sheath [10, 61]. Recently, myelin-associated as well as axonal antigens have been detected in cervical lymph nodes during experimental autoimmune encephalitis (EAE) [11], multiple sclerosis (MS) [12] and after axotomy [37, 59], but it is unclear whether they are drained passively within the cerebrospinal fluid (CSF) or transported actively out of the brain by a sort of dendritic (DC) or monocytic cell. Such transportation would conflict with the predominant current view that antigen-presenting cells (APC) do not leave the parenchyma of the brain. However, cells expressing DC/APC markers have been detected in the meninges, the choroid plexus [30, 33, 34], and the cerebrospinal fluid [41, 42] and they appear in the parenchyma under inflammatory conditions [13, 16, 39, 46, 47, 51, 52, 55]. Recently, it has been shown that the induction of neuroinflammation depends on antigen-presenting dendritic cells associated with brain vessels [16, 32], but their topographic localization and distribution remained unknown. This was at least in part due to the problem that currently available antibodies raised against DC markers such as CD11c and CD205 provided no or questionable signals within the normal mouse brain. Here, we used mice expressing green fluorescent protein (GFP) under the control of the CD11c promoter itgax for an in-depth analysis of the CD11c? population within the central nervous system (CNS) [19]. In line with previous reports, we found numerous ramified CD11c? cells within the meninges and the choroid plexus. However, we report that they also reside within the brain, the spinal cord, and the optic nerve, where they are mostly located within the parenchyma from where they extend processes to the glia limitans. Since re-stimulation of T cells by their cognate antigen in perivascular spaces is indispensable for their progression across the glia limitans [4, 58], it may be the presence of CD11c? cells at the glia limitans rendering them crucial for the onset of neuroinflammation [16]. At present, the origin of the DC observed in (autoimmune) neuroinflammation is unclear. They may either derive from microglia stimulated with granulocyte macrophage colony-stimulating factor (GM-CSF) [49] or infiltrate the parenchyma from the blood along with other leukocytes [16, 39]. Using slice cultures derived from p3 and adult CD11c-GFP mice and chimeras grafted with CD11c-GFP bone marrow, we demonstrate for the first 123 time that CD11c? cells can differentiate from an intraparenchymal precursor in situ and—in principle—can be recruited from the systemic circulation. Due to the irradiation used to create bone marrow chimeras, the latter finding, however, must be interpreted with care since Mildner et al. [36] demonstrated that shielding of brain areas during irradiation specifically blocks infiltration into the respective regions. Finally, we identified a population reflecting the intraparenchymal location of CD11c? cells in human brains using the DC-marker CD209. Materials and methods Mouse strains and housing CD11c-diphtheria toxin receptor (DTR)-GFP transgenic (tg) mice [19], i.e., mice expressing GFP under the control of the CD11c promoter itgax, on a C57BL/6 background were purchased from Charles River (Charles River Laboratories, Inc., Wilmington, MA) and housed under standard conditions with free access to food and water. Care was been taken to minimize any pain and discomfort to animals. All experiments were performed after approval by the Animal Ethical Committee according to German legislation on animal experiments. Perfusion and fixation Animals (n = 30; 7–21 weeks of age) were killed and transcardially perfused with 100 ml 0.9% NaCl (AppliChem. Darmstadt, Germany) followed by 100 ml of a fixative containing 4% paraformaldehyde (PFA) (Merck, Germany) in 0.1 M phosphate buffered saline (PBS; pH 7.4). Brains were removed and post-fixed overnight in the same fixative and cut on a vibratome (30–50 lm sections) (Microm, HM 650V) or incubated in a solution of 30% sucrose (Fluka Biochemika, Germany) in 0.1 M phosphate buffer (PB; pH 7) for 24 h. These sections were embedded in Neg-50 medium (Richard-Allan Scientific, Kalamazoo, MI), snap-frozen in -80°C cold methylbutane (Roth, Karlsruhe, Germany), and cut further into 10–20 lm cryostat sections. For semithin sections and electron microscopy, the fixative contained 4% sucrose and 0.2% glutaraldehyde (AgarScientific, United Kingdom) in carbonate buffered PFA. The brains were removed and postfixed over night in the same solution. Immunostaining for (confocal) fluorescence microscopy For the immunocytochemistry, slices were fixed at 1, 24, 48 and 72 h in 4% paraformaldehyde overnight at 4°C. 33 Acta Neuropathol (2011) 121:445–458 Subsequently, the slices were stored in 0.8 M saccharose-solution over 2 weeks at 4°C. Afterwards, the slices were cut into 10 lm sections with a cryostat. Unspecific binding was blocked using 10% normal goat serum (NGS) (Chemicon, Temecula, CA) and 0.5% Triton X-100 (VWR, Prolabo, Darmstadt, Germany) in PB. After incubation in this solution for 30 min, sections were incubated at ?4°C overnight in PB containing 1% NGS and 0,5% Triton X-100 and the respective primary antibodies: polyclonal anti-ionized calciumbinding adaptor molecule 1 (anti-IBA-1) (1:1,000; rabbit anti mouse; WAKO Chemicals, Neuss, Germany); antiCD11b (1:100; rat anti mouse; Leinco, St. Louis, MO); anti-major histocompatibility complex II (anti-MHC-II) (1:100; rat anti mouse; BD, Heidelberg, Germany); antipan-laminin (1:200; rabbit anti mouse; DakoCytomation, Denmark); anti-glial fibrillary acidic protein (anti-GFAP) (1:1,000; mouse anti mouse; Chemicon, Temecula, CA). After several washes in PB, sections were incubated for 90 min at room temperature in PB containing 1% NGS and 0.5% Triton X-100, and the corresponding secondary antibody (Alexa 350, Alexa 488, Alexa 568, or Alexa 688; all 1:250; Invitrogen, Karlsruhe, Germany). For nuclear staining the Hoechst reagent (Sigma, Steinheim, Germany) was used according to the manufacturer’s protocol. Sections were embedded in fluorescence mounting medium (DakoCytomation, Denmark). Diaminobenzidine (DAB)-staining For DAB staining, slices were incubated in 1% Naborohydride (Sigma, St. Louis, MO) to reduce background fluorescence. Lipids were unhinged by short incubation in an ascending and descending alcohol series (20–30–40–30–20%). Unspecific binding was blocked using 5% bovine serum albumin (BSA) (Biomol, Hamburg, Germany) in PB for 60 min. Sections were subsequently incubated with the primary antibody (polyclonal goat anti-GFP 1:1,000; Acris, Hiddenhausen, Germany) and 1% BSA dissolved in PB at 4°C overnight. Section were then washed several times in PB and incubated in a solution containing 1% BSA and the biotin-coupled secondary antibody (1:200; Vector-Laboratories, Wertheim-Bettingen, Germany) in PB for 90 min at room temperature. Binding of the primary antibody was visualized using an avidin–biotin (ABC) kit (Vector-Laboratories, Wertheim-Bettingen, Germany) with DAB as chromogen according to the manufacturer’s instructions. Slices were dehydrated and embedded in Durcupan (ACM Fluka, Sigma-Aldrich, Gillingham, UK). Semithin and ultrathin sections were cut using a Leica Ultracut (Ultracut UCT, Leica). Organotypic slice cultures Organotypic slice cultures were prepared from either 3-dayold or adult CD11c-DTR-GFPtg mice (Jackson Laboratories, Boston, MA, USA) as previously described [24]. In brief, mice were perfused with saline to eliminate blood leukocytes from the circulation. After decapitation, the brain was rapidly removed under sterile conditions and placed in ice-cold preparation medium consisting of 50% minimum essential medium (MEM) (Gibco, Karlsruhe, Germany), 49 ml aqua pro inject, with 2 mM L-glutamine (Gibco, Karlsruhe, Germany) at pH 7.35. The hippocampi were taken from the brain and cut into 350 lm thick vertical slices on a tissue chopper (Technical Products International, St. Louis, MO, USA). Subsequently, the slices were cultured on Millipore cell culture inserts (pore size 0.4 lm; Millipore, USA) in six-well plates containing cultivation medium. The sterile medium contained 25% MEM, 25% basal medium eagle (BME) (Gibco, Karlsruhe, Germany), 25% heat-inactivated normal horse serum (Gibco), 20.9 ml aqua pro inject, 2 mM L-glutamine, 0.65% glucose-20 (Braun, Melsungen, Germany), at pH 7.2. The organotypic slice cultures were incubated at 37°C in a humidified atmosphere with 5% CO2 for 1, 24, 48 and 72 h. The culture medium was changed every 48 h (n = 10). To exclude that exogenous effects induce the differentiation of dendritic cell in the slice culture, we performed the same experiments with serum-free medium using p3 and adult animals (n = 10). The serum-free medium contained (for 100 ml) 37.5 ml MEM, 20.9 ml aqua pro inject, 37.5 ml BME with 2 mM glutamine and 0.65% glucose-20 (Gibco, Karlsruhe, Germany) at pH 7.2 (n = 3). Irradiation of bone marrow chimeric mice CD11c-GFP mice were killed by cervical dislocation. Bone marrow (BM) cells were isolated by flushing femur and tibia bones with Dulbecco’s Modified Eagle Medium (DMEM) containing 1% penicillin/streptomycin and 1% fetal bovine serum (all from GIBCO, Invitrogen, Karlsruhe, Germany) filtered through a 40 lm cell strainer (BD, Heidelberg, Germany). C57BL/6J wild type (wt) recipient mice were lethally irradiated with 11 Gy (split dose 2 9 5.5 Gy) and intravenously injected with 4–6 9 106 BM cells. On each of days 5, 15, 28, 46, 75, 105 and 161 (15 and 23 weeks; n = 4) after transplantation mice were killed for histological analysis. Entorhinal cortex lesion (ECL) Stereotaxic lesioning of the left entorhinal cortex was performed as described in detail elsewhere [6, 25]. In brief, 123 34 mice were anaesthetized with a mixture of ketamine (20%) (Cura Med, Germany) and Rompun (8%) (Bayer Vital, Germany) (10 ll/g body weight). They were then fixed in an stereotactic apparatus (Kopf Instruments, Tujunga, CA) and the eyes were protected with a humid soft tissue. For ECL, the left entorhinal cortex was lesioned using a 2 mm broad knife. After exposing the skull the following coordinates measured from k (where the longitudinal and the k suture meet) were used: anteroposterior ?0.4 mm, lateral ?1 mm and dorsoventral, down to the base of the skull. The wound was carefully sutured and the animal was placed back in its cage. Three days after entorhinal lesion, animals were killed and perfused as described above. Microscopy To analyze the colocalization of GFP? cells with the expression of common microglia markers and to determine the distribution of the GFP? cells in the slice, a Leica TCS SL laser scanning microscope with was used. 3D-reconstruction was performed with Volocity 3 software (Improvision, Tübingen, Germany) (n = 3). Fluorescent stainings and semithin-sections were studied using an Olympus BX51 fluorescence and light- microscope or a Zeiss Axiovert 100 M LSM510 confocal microscope. For analysis of the 3D-extension of CD11c-GFP cells within slices and the stainings of BM-chimeras, an Olympus Fluoview FV1000 was used. The 3D-reconstruction was performed with Imaris software. Electron-microscopy was performed using a Zeiss EM109. Human tissues Formalin-fixed and paraffin-embedded human autopsy material with or without neuropathological alteration was used. All cases underwent detailed neuropathologic examination. Informed consent for autopsy and subsequent use of tissue for research purposes was given. To mark human DC a primary antibody to human DC-SIGN (CD209; R&D Systems; 1:10 dilution) was used upon ethylenediaminetetraacetic acid (EDTA)-driven antigen retrieval. The immunohistochemical staining was carried out on an automated Benchmark staining apparatus (Ventana Medical Systems/Roche) following the manufacturer’s guidelines. Results Distribution of CD11c-GFP cells throughout the CNS (Fig. 1) Serial sections of brain, spinal cord, and optic nerve revealed a consistent rather than random distribution 123 pattern of CD11c-GFP cells (Fig. 1a). In the brain, they were preferentially situated within the tela of the choroid plexus, beneath the ependyma of the lateral ventricles, along the adjacent corpus callosum and the fornix (Fig. 1b– d). Regularly, but at lower numbers, CD11c-GFP cells were also present in the white matter of the olfactory bulbs, the brain stem and the cerebellum. Such preferential location within the white matter was also evident in the spinal cord (Fig. 1e), where the largest numbers were found along the spinobulbar tract and the lateral fibre tracts, while the spinal nerves were not populated by CD11c-GFP cells (Fig. 1f–h). Importantly, the optic nerve was found to be densely populated by CD11c-GFP cells (Fig. 1i, k), while fluorescent cells were extremely rare within other cranial nerves, e.g. the trigeminal nerve (Fig. 1l) and the trigeminal ganglion. Parenchymal, but not choroid plexus CD11c-GFP cells express microglial markers but lack MHC-II (Fig. 2) At higher magnification CD11c-GFP cells exhibited ramified morphologies with more plump processes in the ependyma of the lateral ventricles and tiny ramifications within fibre tracts. Counterstaining with IBA-1 and CD11b revealed that almost all CD11c-GFP cells expressed these microglial markers. The ratio of CD11c-GFP cells among all microglia stained with IBA-1 or CD11b ranged between none or occasional cells (e.g., cortex) and a maximum of up to two thirds (optic nerve). MHC-II was expressed at detectable levels by CD11c-GFP cells only in the choroid plexus. In contrast to all other regions studied, CD11c-GFP cells of the plexus did not regularly express IBA-1 and CD11b (Fig. 2g–i). Meningeal and perivascular macrophages expressed MHC-II in all brain areas (Fig. 2c–m). CD11c-GFP cells are preferentially localized in the juxtavascular neuropil (Fig. 3) As anticipated from fluorescence microscopy and a previous report [16], CD11c-GFP cells were regularly found in close vicinity to blood vessels (Fig. 3a) raising the question of whether they are part of the vascular wall, reside in perivascular spaces (i.e., the compartment between the vascular basement membrane and the glia limitans) or within the juxtavascular parenchyma (i.e., the neuropil beyond the glia limitans) [4, 40] (Fig. 3b). To address this issue, the borders between these three compartments (vascular wall, perivascular space, and juxtavascular parenchyma) were demarcated using GFAP and/or laminin antibodies. As the vascular and the glial basement membranes differ in their laminin contents [53], an anti-pan-laminin serum was used allowing simultaneous detection of both membranes (Fig. 3d, e). To unequivocally distinguish the perivascular Acta Neuropathol (2011) 121:445–458 35 Fig. 1 Distribution of CD11cGFP cells throughout the CNS. a–d Distribution of CD11c-GFP cells in the forebrain. a Four horizontal brain hematoxylin and eosin-stained sections from different levels schematically show the typical distribution of CD11c-GFP cells. Sites of evident accumulation included the periventricular/ subependymal area, the alveus, fimbria, the choroid plexus, and the corpus callosum. b– d CD11c-GFP cells are shown at sites of typical accumulation in 16 lm cryosections as indicated by frames in a (sections 2 and 3). b Subventricular zone of the frontal pole of the lateral ventricle; c fornix; d choroid plexus/tela choroidea of the third ventricle. e–h Distribution of CD11c-GFP cells in the spinal cord. The frames depicted in e are shown under high magnification in f–h. f The spinothalamic tract is densely populated by CD11c-GFP cells. The white line indicates the surface of the spinal cord. g Many CD11c-GFP cells are regularly found in the spinobulbar tract. h No cells were found in the spinal nerve and its roots, visualized with Hoechst staining. i, k, l While the optic nerve (i, k) is densely populated by CD11c-GFP cells, the trigeminal nerve (l) and other cranial nerves contain only very few fluorescent cells (arrow in l) space from the parenchyma, the localization of identified CD11c-GFP cells was further analysed in semi- and ultrathin sections (1.0 and 0.055 lm, respectively). In laminin-stained samples, most CD11c-GFP cells appeared to reside within the juxtavascular parenchyma (Fig. 3a). This finding was confirmed in semithin sections (Figs. 3c, 4c). Only occasional cells were located within perivascular spaces. In contrast to the parenchymal population, these cells lacked tiny ramifications (Fig. 3d–e). CD11c-GFP cells participate in the glia limitans organization (Fig. 4) Confocal analysis of sections stained for laminin or GFAP suggested that processes of CD11c-GFP cells participate in the formation of the glia limitans (Fig. 4a, b). The same was observed in semithin sections, where GFP was stained with a polyclonal antiserum (Fig. 4c). In fact, electron microscopical analysis revealed that CD11c-GFP cells directly attach to the basement membrane of the glia limitans (Fig. 4d) thus representing an integral element of this glial barrier. Origin of CD11c-GFP cells (I): appearance of CD11c? cells in organotypic brain slices (Figs. 5, 6, 7) The origin of CD11c? dendritic cells in inflamed neural tissue is currently unclear. They may either be recruited from blood or derive from intrinsic (microglial) precursors. To test the latter possibility, we prepared organotypic slice 123 36 Fig. 2 Parenchymal, but not choroid plexus CD11c-GFP cells express microglial markers but lack MHC-II. Brain (a–c), spinal cord (d–f), choroid plexus (g–l) and optic nerve (k–m) of CD11c-GFP mice were counterstained with antibodies for the microglial markers IBA-1 (a, d, g, k) and CD11b (b, e, h, l) and for MHC-II (c, f, i, m). CD11c-GFP is shown in green, IBA-1, CD11b, and MHC-II in red, nuclei stained with Hoechst are shown in blue. The inserts represent one characteristic cell for each staining. Note that the antibody against MHC-II strongly labels meningeal macrophages, but not intraparenchymal GFP-expressing cells. Green arrows in g and h point to CD11cGFP cells, which are negative for either IBA-1 (d) or CD11c (e) cultures obtained from CD11c-GFP mice and searched for GFP? cells in living cultures using an epifluorescence microscope. Despite the relatively low resolution of this mode of observation, it was already evident that the number of GFP-expressing cells strongly increased over time. For a more detailed analysis of the respective cells, slices were subsequently fixed at various time points and cryosections were prepared for immunocytochemistry. As shown in Fig. 5a–c, few or no fluorescent cells were visible in slices from p3 animals at 1 h after incubation. At 24 h, numerous GFP? cells could be detected in all cultures (Fig. 5d–f). These cells were located throughout the whole width of the slices which was evident in cross sections cut perpendicularly to the surface of the slice (Fig. 5f). Cryosections of these slices revealed that the fluorescent cells exhibited ramified morphologies and stained positive for the microglial markers MAC-1 and IBA-1 (Fig. 6). No additional increase of GFP? cells was apparent at 48 and 72 h. An important question was whether the induction of CD11c on cells within slice cultures was restricted to the early postnatal phase. Therefore, we prepared acute brain slices from adult (8–12 weeks old) mice and studied the appearance of fluorescent cells in these tissues. Again, fluorescent cells were rare or absent directly after incubation (Fig. 5g–i), but numerous cells were found after 24 h 123 Acta Neuropathol (2011) 121:445–458 37 Fig. 3 CD11c-GFP cells are preferentially localized in the juxtavascular neuropil. a Confocal 3D-reconstruction of CD11c-GFP cells and their relation to a brain vessel (stained for pan-laminin in red) in a 50 lm vibratome section. As described previously [16], all cells are to be vessel-associated raising the question of whether they are in the juxtavascular parenchyma or in the perivascular space. Both compartments seem to be populated (arrowhead juxtavascular, arrow perivascular), but it is impossible to unequivocally distinguish between the two in these sections. b Schematic illustration of the different compartments of the neurovascular interface. The vascular wall represents the first compartment and consists of endothelial cells, pericytes and (if present) smooth muscle cells of the tunica media. The second is the perivascular (Virchow/Robin) space which harbors perivascular macrophages and leptomeningeal mesothelial cells. It is on the one side bordered by the basement membrane of the vascular wall, and by the basement membrane of the glia limitans on the other. The third compartment is the juxtavascular parenchyma starting beyond the astrocytic endfeet forming the glia limitans. c The existence of juxtavascular, and thus intraparenchymal CD11c-GFP cells was confirmed in semi-thin sections (1.0 lm) in which GFP was visualized with an antibody and DAB staining. The neuropil is counterstained with toluidine-blue. The vast majority of CD11c-GFP cells turned out to be located within the juxtavascular parenchyma. d, e In addition, scattered individual cells cells were also found in perivascular spaces depicted either by laminin staining (red) to show the outer vascular and parenchymal basement membranes (arrows) and thus the perivascular space in d or by combined laminin (red) and GFAP (blue) staining. The insert in d shows the same cell after cutting for semithin analysis which clearly confirms its location within the perivascular space (arrows point to basement membranes) in vitro (Fig. 5j–l). Thus, just like in the early postnatal phase, adult brain tissue apparently contains a cell type with the potential to express CD11c. As shown in Fig. 7, 3D-reconstruction of the CD11cGFP cells revealed tiny extensions of up to 35 lm with rare or invisible secondary ramifications. Due to the counterstaining of nuclei with Hoechst, it also became apparent that the cells were clearly located within the parenchyma rather than being part of (peri-)vascular remnants (Fig. 7). 123 38 Fig. 4 CD11c-GFP cells participate in the glia limitans organization. As shown in Fig. 3a and c, some cells seemed to participate in the organization of the glia limitans. a This was confirmed by triple fluorescence analysis depicting GFAP (blue), laminin (red), and GFP (green). Note that the processes of CD11c-GFP cells intermingle with the astrocytic endfeet of the glia limitans. b This was also evident in sections cutting vessels perpendicularly to their longitudinal axis, where CD11cGFP processes were found to embrace the parenchymal basement membrane (stained with laminin in red, nuclei blue). The insert shows a confocal picture in which CD11c-GFP processes appeared to be directly attached to the parenchymal basement membrane. c–d This was confirmed in semithin sections (c) and ultrastructural analysis (original magnification 94,400) (d) of GFP-DAB-stained sections, where the direct contact (green arrows) between GFP-filled cells and the basement membrane (dotted red line) could be proven Fig. 5 Overviews of CD11c-GFP cells in slice cultures. Organotypic slice cultures were prepared from either p3 (a–f) or adult (g–l) CD11c-DTR-GFPtg mice and kept in culture for either 1 h (a–c, g–i) or 24 h (d–f, j–l). CD11c-GFP-expressing cells were rare or absent at 24 h in p3 (a–c) and adult (g–h) slices. At 24 h, however, fluorescent cells were abundant in all slices irrespective of whether 123 they derived from p3 (d–e) or adult (j–l) mice (n = 10). As a proof of principle, these data demonstrate that neural tissue harbors a cell type which can be induced to express CD11c. Thus, recruitment across the blood–brain barrier is not an absolute prerequisite for such cells to appear in the parenchyma 39 Acta Neuropathol (2011) 121:445–458 Fig. 6 Expression of microglial markers on CD11c-GFP cells. Immunocytochemistry was performed in cryosections from slice cultures of all groups, constantly revealing that CD11c-GFP cells were immune-positive for MAC-1 (a–c) and IBA-1 (d–f). Pictures shown here derive from an untreated, p3-slice kept in culture for 24 h. Note that MAC-1 (b) is a membrane protein (CD11b), while IBA-1 (e) and GFP (a, d) are located within the cytoplasm. The same observations were made in slices incubated for 48 and 72 h (n = 8) Fig. 7 3D-reconstruction of parenchymal CD11c-GFP cells. Various CD11c-GFP cells in a slice kept in serum-free medium for 24 h. Nuclei are counterstained with Hoechst. The frames in the upper panel are shown at higher magnification and in 3Dreconstruction in a–c. a A cell showing cardinal branches, but no secondary ramifications, reflecting a typical morphological feature of the de novo appearing CD11c-GFP population. b Arrowheads point to long and tiny extensions. c Arrowheads point to putative remnants of small vessels. The fluorescent cells are clearly located within the adjacent parenchyma rather than in the vascular wall or the perivascular space Origin of CD11c-GFP cells (II): CD11c-GFP cells can be recruited from BM (Fig. 8) In order to test whether the intraparenchymal population of CD11c-GFP cells can also derive from a precursor of the BM, C57BL/6J wt mice were lethally irradiated and transplanted with BM from CD11c-GFP mice. Mice were killed on each of days 5, 15, 28, 46, 75, 105 and 161 (15 and 23 weeks; n = 4) after transplantation. First intraparenchymal CD11c-GFP cells were detected within the periaqueductal white matter at 46 days after transplantation. Subsequently, fornices, brainstem, spinal cord, and optic nerve were populated. Confocal analyses confirmed the intraparenchymal localization using laminin to distinguish perivascular spaces from the neuropil and demonstrated the coexpression of microglia/macrophage markers IBA-1 and CD11b (nearly all cells) and MHC-II (occasional). The number of CD11c-GFP cells is not increased in zones of anterograde axonal degeneration (Fig. 9) We have previously demonstrated that axonal injury induced by entorhinal cortex lesion (ECL) causes induction 123 40 Fig. 8 CD11c-GFP cells can be recruited from BM. Within a time period of 48 days to 23 weeks after transplantation of transgenic CD11c-GFP BM into lethally irradiated wt mice, an increasing number of ramified CD11c-GFP cells was found in characteristic locations within the neuropil surrounding the aqueduct (a), brain stem, fornix (b), fibre tracts surrounding the striatum, white matter of the spinal cord (c) and the optic nerve (d). Counterstaining of the basal laminae with laminin (e) demonstrates the intraparenchymal localization of the CD11c-GFP cells. Further confocal analysis reveals their regular expression of CD11b (f) and IBA-1 (g) (orange arrows) and their occasional expression of MHC-II (h) Fig. 9 The number of CD11c-GFP cells is not increased in zones of anterograde axonal degeneration. We have previously shown that myelin-phagocytosing cells in zones of axonal degeneration induced by entorhinal cortex lesion (ECL) express MHC-II and CD86 [7], and that mononuclear cells are recruited which subsequently transform into microglia [5]. a–b To test whether these cells also express CD11c, we performed ECL in adult mice. The distribution of microglia in the normal hippocampus is shown in b. A massive accumulation of IBA-1 (red) cells is visible in the zones of axonal degeneration in the hippocampus, the molecular layer (ML). However, these cells did not express CD11c. The frame depicts a CD11cGFP cell located at a vessel in the entorhinal cortex which is shown at higher magnification in the insert of CD86 and MHC-II on microglia-like elements [7] as well as the recruitment of hematogenous cells and their subsequent transformation into microglia [5]. Similar findings have been reported by others [2, 26, 62, 63]. Therefore, we speculated that CD11c expression may also be induced on local microglia or CD11c? hematogeneous cells may be recruited after ECL. However, an increase in neither number nor distribution of CD11c-GFP cells was evident in this setting (Fig. 9a, b). Thus, although axonal degeneration provides cues for the recruitment of mononuclear cells from the blood and cells expressing the stem cell marker CD34 [26], CD11c is not found on these populations. 123 Anti-CD209 demarks a population of cells in human brains the distribution of which reflects intraparenchymal mouse CD11c cells (Fig. 10) As shown above, CD11c-GFP cells do not only populate the meninges, the choroid plexus and the perivascular space, but also reside within the brain parenchyma (Figs. 1, 2, 3, 4). A similar population was identified in human Acta Neuropathol (2011) 121:445–458 41 Fig. 10 In the human brain parenchymal DC are present in the periventricular area. To analyze the distribution of DC in human paraffin slices of different human brains were stained with CD209, an established human DC-marker. In addition to many perivascular cells, that were described earlier, a population of juxtavascular DC reflecting the intraparenchymal location of the CD11c? cells of the CD11cGFPtg-mice (Figs. 1, 2, 3, 4) could be detected in the periventricular regions of the human brains. a– b Periventricular white matter. c Optical nerve (c derives from a patient with elective necrosis of the cortex and hippocampus, a and d from an individual without brain pathology) brains using the DC-marker CD209. These cells were also located adjacent to blood vessels clearly beyond the glia limitans within the parenchyma (Fig. 10). Discussion In continuation of the work by Hickey and Kimura [18], two recent papers demonstrate that a population of brainbased antigen-presenting, CD11c? cells are required and sufficient to stimulate autoimmune neuroinflammation within the CNS [16, 32]. Both reports demonstrated that the population of DC-like elements with the functional capacity to support T cell activation derives from the systemic myeloid compartment and not from the CNSparenchyma, but it was unknown whether they reside in perivascular spaces or within the parenchyma. Here, we explored the distribution and localization of such CD11c? cells within the normal mouse brain, spinal cord and optic nerve. To this end, we took advantage of a transgenic mouse strain expressing GFP under the itgax promoter, since all available antibodies to mouse CD11c reveal weak, questionable or unspecific staining in cryo-, vibratom-, or paraffin sections of the brain. This may be explained by low expression levels of the respective markers under normal conditions, which may be driven by factors exclusively present in the normal parenchyma, because DC were found in the leptomeningeal compartment and during neuroinflammation using the same antibodies [13, 16, 21, 30, 33, 51] (for review see [42]). We found that (a) CD11c-GFP cells reside in virtually all regions of the brain and the spinal cord, (b) they are not randomly scattered, but their distribution shows a typical pattern with preferential localization in the periventricular areas and the adjacent fibre tracts as well as in the optic nerve and the white matter of the spinal cord, (c) only few cells are located in perivascular spaces, while the vast majority resides in the juxtavascular parenchyma from where they extend processes to the basement membrane of the glia limitans, (d) CD11c can be induced in cells already present in the brain, but CD11c cells are also recruited from the blood, (e) CD209 labels an intraparenchymal population of cells in human brains with features similar to the CD11c-GFP cells described herein. We regard it as the main finding that CD11c-GFP cells are located within the parenchyma, but extend processes to the glia limitans. We have previously proposed that neuroinflammation involves two differentially regulated steps: (1) passage of the vascular wall into perivascular spaces, and (2) progression across the glia limitans into the 123 42 neuropil [4, 40]. While the first step has been investigated intensively (for review: [23, 27], much less is known about the second. The group of Owens was the first to experimentally dissect steps one and two: they found that elimination of perivascular cells and subsequent induction of EAE does not interfere with perivascular accumulation of leukocytes, but completely blocks their progression across the glia limitans [58]. The same group demonstrated that the chemokine CCL2 is a major player in driving recruitment of leukocytes into the parenchyma [2]. In mice overexpressing CCL2 in the brain, i.p. application of pertussis toxin initiates cellular infiltration across the glia limitans causing weight loss and encephalopathy. Strikingly, treatment of these mice with the broad spectrum metalloproteinase (MMP) inhibitor BB-94/Batimastat did not interfere with perivascular infiltration either, but instead abolished the second step along with clinical symptoms [57]. Others showed that MMP-2/MMP-9 double knock-out mice are resistant to EAE, because they cannot cleave dystroglycan, which anchors astrocytic endfeet to the basement membrane of the glia limitans [1]. These studies strongly suggest that expression of MMPs by either the T cells or macrophages in the perivascular space is a prerequisite for passage of the glia limitans and the induction of symptomatic neuroinflammation in EAE. However, if animals are immunized with horseradish peroxidase (HRP) and the same antigen is subsequently injected into the ventricle, T cells home to HRP-phagocytosing perivascular macrophages upon peripheral antigen boosting, but they do not pass the glia limitans. Again, this T cell extravasation over the first barrier provided by the endothelium does not cause clinical symptoms [60]. All these studies are integrated by the view that antigenpresentation/recognition in the perivascular space may be necessary, but per se are not sufficient for the induction of intraparenchymal neuroinflammation [60]. The second step of neuroinflammation may require presentation of an intraparenchymal antigen at the glia limitans itself. In fact, non-astrocytic processes immune positive for microglial markers have been demonstrated at the ultrastructural level by Lassmann [28]. It has often been questioned how T cells can be re-stimulated within the brain upon adoptive transfer to cause passive EAE, since their cognate antigen should not normally be present within perivascular macrophages. Given that the decisive antigen-presenting cell type expresses CD11c [16, 32] and, as shown here, is located at the glia limitans, one can picture a scenario in which, e.g., physiological degradation of myelin and uptake by local CD-11c? cells causes permanent presentation of its epitopes at the interface between parenchyma and perivascular space. The arrival of encephalitogenic Th1/Th17 cells and the accompanying secretion of proinflammatory cytokines could then induce maturation of 123 CD11c? antigen-presenting cells eventually leading to MMP expression and permeabilization of the glia limitans. Thus, the localization of CD11? cells at the glia limitans provides a possible explanation for their recently described crucial function in autoimmune neuroinflammation [16] and raises the possibility that alterations in their recruitment, development, or activation state represents an early step in the pathogenesis of autoimmune diseases such as MS. Our data clearly demonstrate the presence of ramified cells expressing CD11c and several markers shared with, but not specific for microglia. The question remains whether these cells should be regarded as DC. CD11c is a membrane glycoprotein which belongs to the CD18 family of integrins, including CD11a, CD11b, and CD11d. Functionally, CD11c is responsible for binding a diverse array of ligands such as endothelial cell adhesion molecules, bacterial cell wall components, complement factors such as iC3b, and matrix proteins [20, 29, 38, 48, 50, 56]. In vivo, CD11c expression is also induced during early stages of myeloid cell differentiation [20] and is increased considerably during differentiation of monocytes into tissue macrophages [50]. CD11c expression is mostly restricted to cells of the myeloid lineage: macrophages, monocytes, granulocytes, and at characteristically much higher levels—most DC express CD11c, but a small population of CD11c? B and T lymphocytes (activated B and cytotoxic T cells) has also been described [22, 23, 27, 44, 54]. Thus, the cell type demonstrated herein can either be viewed as a subpopulation of microglia, which belongs to the monocytic lineage, or might represent a form of immature, quiescent, or tolerogenic DC which lack the expression of MHC-II. Beyond pure terminology, the latter would imply the capability of these cells to carry antigens from the CNS parenchyma into the draining cervical and lumbar lymph nodes and other lymphoid organs. Such migration has only been observed upon injection of DC [9, 17, 21] and seems to involve the cribriform plate as major exit route from brain to nasal mucosa [15]. However, at present, direct evidence for a cell type carrying antigens from the brain to lymphoid organs is lacking. Our data unequivocally demonstrate the presence of CD11c? cells with ramified morphology in the parenchyma of normal adult mouse brain, spinal cord and optic nerve and of a similar population in man. Their extensions into the glia limitans provide a possible explanation of how intraparenchymal myelin can be presented to adoptively transferred T cells. Of note, we found MHC-II expressing CD11? cells in the choroid plexus, which may be involved in the previously reported CCR6-dependent recruitment of Th17 cells into the brain [45]. This route would also be in line with our observation that CD11c? cells reside in the ependyma through which T cells could be guided from the 43 Acta Neuropathol (2011) 121:445–458 ventricles. Studying changes in the CD11? population under neuroinflammatory conditions and subsequently relating them to MS lesions could provide very useful information on the development, chronicity, and progression of MS. Acknowledgments This study was supported by the DFG (FoGr ‘‘Fate of barin macrophages’’ to I.B.), the Dutch MS Research Foundation (JDL) and the COST consortium NEURINFNET (BM0603: J.D.L., B.B., I.B.). The Olympus Fluoview microscope was financed by the Kassel-Stiftung, the Messer-Stiftung, and the Dr. Senckenberg-Stiftung. We gratefully acknowledge the help of these Frankfurt foundations. I.B. is a fellow of the Frankfurt Institute of Advanced Studies (FIAS). 16. 17. 18. 19. 20. References 21. 1. Agrawal S, Anderson P, Durbeej M et al (2006) Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med 203:1007–1019 2. Babcock AA, Kuziel WA, Rivest S, Owens T (2003) Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci 23:7922–7930 3. Bechmann I (2005) Failed central nervous system regeneration: a downside of immune privilege? Neuromolecular Med 7:217–228 4. Bechmann I, Galea I, Perry VH (2007) What is the blood–brain barrier (not)? Trends Immunol 28:5–11 5. Bechmann I, Goldmann J, Kovac AD et al (2005) Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. FASEB J 19:647–649 6. Bechmann I, Nitsch R (1997) Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique. Glia 20:145–154 7. Bechmann I, Peter S, Beyer M, Gimsa U, Nitsch R (2001) Presence of B7–2 (CD86) and lack of B7–1 (CD(80) on myelin phagocytosing MHC-II-positive rat microglia is associated with nondestructive immunity in vivo. FASEB J 15:1086–1088 8. Billingham RE, Boswell T (1953) Studies on the problem of corneal homografts. Proc R Soc Lond B Biol Sci 141:392–406 9. Carson MJ, Reilly CR, Sutcliffe JG, Lo D (1999) Disproportionate recruitment of CD8? T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol 154:481–494 10. Cserr HF, Knopf PM (1992) Cervical lymphatics, the blood–brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 13:507–512 11. de Vos AF, van Meurs M, Brok HP et al (2002) Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol 169:5415–5423 12. Fabriek BO, Zwemmer JN, Teunissen CE et al (2005) In vivo detection of myelin proteins in cervical lymph nodes of MS patients using ultrasound-guided fine-needle aspiration cytology. J Neuroimmunol 161:190–194 13. Fischer HG, Reichmann G (2001) Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol 166:2717–2726 14. Galea I, Bechmann I, Perry VH (2007) What is immune privilege (not)? Trends Immunol 28:12–18 15. Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I (2006) T cells traffic from brain to cervical lymph 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol 80:797–801 Greter M, Heppner FL, Lemos MP et al (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11:328–334 Hatterer E, Davoust N, Didier-Bazes M et al (2006) How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood 107:806–812 Hickey WF, Kimura H (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239:290–292 Jung S, Unutmaz D, Wong P et al (2002) In vivo depletion of CD11c(?) dendritic cells abrogates priming of CD8(?) T cells by exogenous cell-associated antigens. Immunity 17:211–220 Kansas GS, Muirhead MJ, Dailey MO (1990) Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood 76:2483–2492 Karman J, Chu HH, Co DO, Seroogy CM, Sandor M, Fabry Z (2006) Dendritic cells amplify T cell-mediated immune responses in the central nervous system. J Immunol 177:7750–7760 Keizer GD, Borst J, Visser W, Schwarting R, de Vries JE, Figdor CG (1987) Membrane glycoprotein p150, 95 of human cytotoxic T cell clone is involved in conjugate formation with target cells. J Immunol 138:3130–3136 Kishimoto TK, Larson RS, Corbi AL, Dustin ML, Staunton DE, Springer TA (1989) The leukocyte integrins. Adv Immunol 46:149–182 Kluge A, Hailer NP, Horvath TL, Bechmann I, Nitsch R (1998) Tracing of the entorhinal-hippocampal pathway in vitro. Hippocampus 8:57–68 Kovac AD, Kwidzinski E, Heimrich BI et al (2004) Entorhinal cortex lesion in the mouse induces transsynaptic death of perforant path target neurons. Brain Pathol 14:249–257 Ladeby R, Wirenfeldt M, Dalmau I et al (2005) Proliferating resident microglia express the stem cell antigen CD34 in response to acute neural injury. Glia 50:121–131 Larson RS, Springer TA (1990) Structure and function of leukocyte integrins. Immunol Rev 114:181–217 Lassmann H, Zimprich F, Vass K, Hickey WF (1991) Microglial cells are a component of the perivascular glia limitans. J Neurosci Res 28(2):236–243 Lopez-Cabrera M, Nueda A, Vara A, Garcia-Aguilar J, Tugores A, Corbi AL (1993) Characterization of the p150, 95 leukocyte integrin alpha subunit (CD11c) gene promoter. Identification of cis-acting elements. J Biol Chem 268:1187–1193 Matyszak MK, Perry VH (1996) The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience 74:599–608 Matyszak MK, Perry VH (1998) Bacillus Calmette-Guerin sequestered in the brain parenchyma escapes immune recognition. J Neuroimmunol 82:73–80 McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD (2005) Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11:335–339 McMenamin PG (1999) Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol 405:553–562 McMenamin PG, Wealthall RJ, Deverall M, Cooper SJ, Griffin B (2003) Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res 313:259–269 123 44 35. Medawar PB (1948) Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29:58–69 36. Mildner A, Schmidt H, Nitsche M et al (2007) Microglia in the adult brain arise from Ly-6ChiCCR2? monocytes only under defined host conditions. Nat Neurosci 10(12):1544–1553 37. Mutlu L, Brandt C, Kwidzinski E et al (2007) Tolerogenic effect of fiber tract injury: reduced EAE severity following entorhinal cortex lesion. Exp Brain Res 178:542–553 38. Myones BL, Dalzell JG, Hogg N, Ross GD (1988) Neutrophil and monocyte cell surface p150, 95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest 82:640–651 39. Newman TA, Galea I, van Rooijen N, Perry VH (2005) Bloodderived dendritic cells in an acute brain injury. J Neuroimmunol 166:167–172 40. Owens T, Bechmann I, Engelhardt B (2008) Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 67:1113–1121 41. Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H (2001) Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain 124:480–492 42. Pashenkov M, Teleshova N, Link H (2003) Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol 13:23–33 43. Perry VH (2000) Persistent pathogens in the parenchyma of the brain. J Neurovirol 6(Suppl):86–89 44. Postigo AA, Corbi AL, Sanchez-Madrid F, de Landazuri MO (1991) Regulated expression and function of CD11c/CD18 integrin on human B lymphocytes. Relation between attachment to fibrinogen and triggering of proliferation through CD11c/CD18. J Exp Med 174:1313–1322 45. Reboldi A, Coisne C, Baumjohann D et al (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10:514–523 46. Reichmann G, Schroeter M, Jander S, Fischer HG (2002) Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. J Neuroimmunol 129:125–132 47. Rosicarelli B, Serafini B, Sbriccoli M et al (2005) Migration of dendritic cells into the brain in a mouse model of prion disease. J Neuroimmunol 165:114–120 48. Sadhu C, Ting HJ, Lipsky B et al (2007) CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J Leukoc Biol 81:1395–1403 49. Santambrogio L, Belyanskaya SL, Fischer FR et al (2001) Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA 98:6295–6300 50. Schwarting R, Stein H, Wang CY (1985) The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood 65:974–983 123 51. Serafini B, Columba-Cabezas S, Di RF, Aloisi F (2000) Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am J Pathol 157:1991–2002 52. Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14:164–174 53. Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM (2001) Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood– brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol 153:933–946 54. Stacker SA, Springer TA (1991) Leukocyte integrin P150, 95 (CD11c/CD18) functions as an adhesion molecule binding to a counter-receptor on stimulated endothelium. J Immunol 146: 648–655 55. Suter T, Biollaz G, Gatto D et al (2003) The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur J Immunol 33:2998–3006 56. Te Velde AA, Keizer GD, Figdor CG (1987) Differential function of LFA-1 family molecules (CD11 and CD18) in adhesion of human monocytes to melanoma and endothelial cells. Immunology 61:261–267 57. Toft-Hansen H, Buist R, Sun XJ, Schellenberg A, Peeling J, Owens T (2006) Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J Immunol 177:7242–7249 58. Tran EH, Hoekstra K, van Roojien N, Dijkstra CD, Owens T (1998) Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol 161:3767–3775 59. van Zwam M, Huizinga R, Melief MJ et al (2009) Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med 87:273–286 60. Walther M, Popratiloff A, Lachnit N et al (2001) Exogenous antigen containing perivascular phagocytes induce a non-encephalitogenic extravasation of primed lymphocytes. J Neuroimmunol 117:30–42 61. Weller RO, Subash M, Preston SD, Mazanti I, Carare RO (2008) Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 18:253–266 62. Wirenfeldt M, Babcock AA, Ladeby R et al (2005) Reactive microgliosis engages distinct responses by microglial subpopulations after minor central nervous system injury. J Neurosci Res 82:507–514 63. Wirenfeldt M, Dissing-Olesen L, Anne BA et al (2007) Population control of resident and immigrant microglia by mitosis and apoptosis. Am J Pathol 171:617–631 45 4.2 CD11c-positive cells from brain, spleen, lung, and liver exhibit site-specific immune phenotypes and plastically adapt to new environments Kerstin Immig, Martin Gericke, Franziska Menzel, Felizitas Merz, Martin Krueger, Fridtjof Schiefenhövel, Andreas Lösche, Kathrin Jäger, Uwe-Karsten Hanisch, Knut Biber K, Ingo Bechmann K. Immig, M. Gericke, F. Menzel, F. Merz, M. Krueger, F. Schiefenhövel, K. Bechmann Institute of Anatomy, University of Leipzig, 04103 Leipzig, Germany A. Lösche, K. Jäger IZKF-FACS-Core Unit, Leipzig University, 04103 Leipzig, Germany Uwe-Karsten Hanisch Institute of Neuropathology, University of Göttingen, 37075 Göttingen, Germany Knut Biber Department of Psychiatry and Psychotherapy, Section of Molecular Psychiatry, University of Freiburg, 79104 Freiburg, Germany 46 RESEARCH ARTICLE CD11c-Positive Cells from Brain, Spleen, Lung, and Liver Exhibit Site-Specific Immune Phenotypes and Plastically Adapt to New Environments Kerstin Immig,1 Martin Gericke,1 Franziska Menzel,1 Felicitas Merz,1 Martin Krueger,1 Fridtjof Schiefenh€ ovel,1 Andreas L€ osche,2 Kathrin J€ ager,2 Uwe-Karsten Hanisch,3 Knut Biber,4 and Ingo Bechmann1 The brain’s immune privilege has been also attributed to the lack of dendritic cells (DC) within its parenchyma and the adjacent meninges, an assumption, which implies maintenance of antigens rather than their presentation in lymphoid organs. Using mice transcribing the green fluorescent protein under the promoter of the DC marker CD11c (itgax), we identified a juxtavascular population of cells expressing this DC marker and demonstrated their origin from bone marrow and local microglia. We now phenotypically compared this population with CD11c/CD45 double-positive cells from lung, liver, and spleen in healthy mice using seven-color flow cytometry. We identified unique, site-specific expression patterns of F4/80, CD80, CD86, CX3CR1, CCR2, FLT3, CD103, and MHC-II. Furthermore, we observed the two known CD45-positive populations (CD45high and CD45int) in the brain, whereas liver, lung, and spleen exhibited a homogeneous CD45high population. CD11c-positive microglia lacked MHC-II expression and CD45high/CD11c-positive cells from the brain have a lower percentage of MHC-IIpositive cells. To test whether phenotypical differences are fixed by origin or specifically develop due to environmental factors, we transplanted brain and spleen mononuclear cells on organotypic slice cultures from brain (OHSC) and spleen (OSSC). We demonstrate that adaption and ramification of MHC-II-positive splenocytes is paralleled by down-regulation of MHC-II, whereas brain-derived mononuclear cells neither ramified nor up-regulated MHC-II in OSSCs. Thus, brain-derived mononuclear cells maintain their MHC-II-negative phenotype within the environment of an immune organ. Intraparenchymal CD11cpositive cells share immunophenotypical characteristics of DCs from other organs but remain unique for their low MHC-II expression. GLIA 2015;63:611–625 Key words: Key words CD11c-positive cells, immune privilege, microglia Introduction D endritic cells (DC) were first described in 1973 (Steinman and Cohn, 2007) and represent key players in the initiation of the innate and adaptive immune responses. They are specialized antigen-processing and antigen-presenting cells (APCs), which are capable of migration to T-cell zones where they present antigens and activate na€ıve T-cells during infection (Steinman et al., 2003b). Under steady state conditions, immature DCs are important for peripheral tolerance, deletion of T-cells (Mohammad et al., 2012; Steinman et al., 2003a) or expansion of regulatory T-cells (Proietto et al., 2008). Different subsets of DCs were described in lymphoid tissues (lymph nodes and spleen) and nonlymphoid organs, such as liver and lung (Mesnil et al., 2012; Nakamoto et al., 2012; van Rijt, 2005). However, besides their function, murine DCs are mainly distinguished by their expression of View this article online at wileyonlinelibrary.com. DOI: 10.1002/glia.22771 Published online December 3, 2014 in Wiley Online Library (wileyonlinelibrary.com). Received Sep 3, 2014, Accepted for publication Nov 6, 2014. Address correspondence to Ingo Bechmann, Institute of Anatomy, Medical Faculty, Leipzig University, Liebigstraße 13, 04103, Leipzig, Germany. E-mail: [email protected] From the 1Institute of Anatomy, Leipzig University, Leipzig, Germany; 2IZKF-FACS-Core Unit, Leipzig University, Leipzig, Germany; 3Institute of Neuropathology, University of G€ ottingen, G€ ottingen, Germany; 4Department of Psychiatry and Psychotherapy, Section of Molecular Psychiatry, University of Freiburg, Freiburg, Germany Additional Supporting Information may be found in the online version of this article. C V 47 CD11c and major histocompatibility complex (MHC) II (Bulloch et al., 2008; Gottfried-Blackmore et al., 2009; Greter et al., 2005; Jung et al., 2002; Lv et al., 2011; McMahon et al., 2005; van Rijt, 2005). The brain‘s immune privilege was originally related to the lack of DCs and a complete isolation of the brain from the peripheral immune system. However, this assumption was challenged by the discovery of DCs in the meninges and choroid plexus in healthy rat and mouse brain (Matyszak and Perry, 1996; McMenamin, 1999; McMenamin et al., 2003). In these studies, DCs and macrophages were identified by expression patterns of MHC-II/OX62 and CD11b/OX42. In addition, the detection of central nervous system (CNS) derived antigens in cervical lymph nodes (CLN) in multiple sclerosis (MS) patients (van Zwam et al., 2009) or in mice after induction of primary oligodendrocyte death (Locatelli et al., 2012) and the observation that brain leukocytes can leave the skull to reach CLNs (Goldmann et al., 2006; Kaminski et al., 2012) challenged the concept of a strict isolation of the CNS from the peripheral immune system. Later, the surface antigen CD11c became a widely used tool to identify murine DCs and to characterize their activity. However, based on the problem that immunolabeling via available antibodies for DC markers like CD11c only provided questionable results under healthy conditions in lymphoid tissue and mouse brain, CD11c-positive DCs were only investigated under inflammatory conditions. This issue was addressed by developing two transgenic mouse strains. First, a transgenic mouse expressing a fusion protein of simian diphtheria toxin receptor (DTR) and GFP under the promoter of CD11c, itgax (Jung et al., 2002), was developed. Later a transgenic Abbreviations APC bm CLN CNS CSFR-1 d DC DTR EAE EYFP gfp IB4 IBA-1 jv LN MFI MHC-II MS NIH OHSC OSSC pv wt antigen presenting cell bone marrow cervical lymph node central nervous system colony stimulating factor 1 receptor day dendritic cell diphtheria toxin experimental autoimmune encephalomyelitis enhanced yellow fluorescent protein green fluorescent protein isolectin B4 calcium-binding adaptor protein 1 juxtavascular lymph node mean fluorescence intensity major histocompatibility complex II multiple sclerosis National Institute of Health organotypic hippocampal slice cultures organotypic spleen slice cultures perivascular wild type mouse expressing the enhanced yellow fluorescent protein (EYFP) under the promoter of CD11c was generated (Lindquist et al., 2004). For the first time, these models allowed differential analyses to investigate the distribution of CD11cpositive cells in the brain under steady-state conditions (Anandasabapathy et al., 2011; Bulloch et al., 2008; Prodinger et al., 2011). In this context, our group identified an intraparenchymal, juxtavascular (jv) population of CD11cpositive cells by immunolabeling for laminin and electron microscopy (Prodinger et al., 2011). Furthermore, we showed the co-expression of these CD11c-positive cells with common microglia markers, such as the calcium-binding adaptor protein 1 (IBA-1) and CD11b (Prodinger et al., 2011). Studies on the nature of DC and macrophagesubpopulations in different nonlymphoid tissues (lung, liver, gut) recently re-initiated the discussion whether DCs can be clearly differentiated from macrophages and whether DCs really represent a unique cell type or rather exist as “a heterogeneous subset of mononuclear cells” (Geissmann et al., 2010; Hume, 2008; Hume et al., 2013; Randolph and Merad, 2013). Confusion may further arise from the fact that studies differentiating DCs from macrophages often neglected the important issue of whether or not these cells were isolated from lymphoid or nonlymphoid tissues. In fact, the expression patterns of both cell types for crucial markers, such as MHC-II, F4/80, CD11b, and CD11c, may not necessarily be comparable between different tissues (Geissmann et al., 2010). In various studies, DCs are distinguished from other populations by their expression of CD11c and MHC-II. In contrast, macrophages are frequently characterized by their expression of CD11b and F4/80, although they are CD11c negative (Bulloch et al., 2008; Greter et al., 2005; Ji et al., 2013; Jung et al., 2002; McMahon et al., 2005). However, CD11c-positive cells from lung, liver, and spleen, which are positive for F4/80 and CD11b were regarded as DCs. Further, cells addressed as macrophages in the brain were shown to express CD11c (McMahon et al., 2005). Outside of the CNS, mononuclear phagocytes isolated from the gut lamina propria express F4/80, colony stimulating factor 1 receptor (CSFR-1), and CD11c, and were thus classified as DCs (Bogunovic et al., 2009; Denning et al., 2007). Further, application of DT in CD11c-DTR-GFP mice did not exclusively lead to a depletion of DCs, but also targeted macrophages in lung, spleen, and LN (Probst et al., 2005; van Rijt, 2005). Although CD11c-positive cells are known to induce T-cell responses, this capacity was also demonstrated for macrophages in animal models for hepatitis and experimental autoimmune encephalomyelitis (EAE) (Mesnil et al., 2012; Nakamoto et al., 2012). Taken together, there is no consent to a uniform definition for DCs, and criteria are often either Volume 63, No. 4 48 Immig et al: Comparison of CD11c1 Microglia based on presence and absence of surface markers or solely relying on functional features, such as activation of na€ıve Tcells, migration to lymphoid organs, and antigen-presentation (Geissmann et al., 2010; Hume, 2008; Hume et al., 2013; McMahon et al., 2005; Randolph and Merad, 2013). In the CNS, microglia are regarded as a population of resident macrophages, which are characterized by their expression of CD45 intermediate (CD45int), IBA-1, and CD11b (Carson et al., 1998; Sedgwick et al., 1991). In the past, these cells were regarded as a bone marrow (bm) derived population (Bechmann et al., 2005). However, later it has been demonstrated that this microglia engraftment during adulthood arises only under specific host endogenous factors (Mildner et al., 2007). Subsequent studies demonstrated that adult microglia rather represent an ontogenetically distinct population in the mononuclear phagocyte system, which is derived from progenitors of the yolk sac (Ginhoux et al., 2010; Kierdorf et al., 2013). As mentioned above, the intraparenchymal CD11c-positive cells have also been shown to express macrophage markers. In this context, CX3CR1 and CCR2 represent important markers to distinguish between the resident microglial population and inflammatory, bloodderived mononuclear phagocytes (Geissmann et al., 2003; Harrison et al., 1998; Jung et al., 2000; Lewis et al., 2011; Niess, 2005; Prinz and Priller, 2010). Because it was shown that the DC marker CD11c is also expressed on macrophages (McMahon et al., 2005; Probst et al., 2005; van Rijt, 2005), and the discussion raised if DCs can be clearly differentiated from macrophages (Geissmann et al., 2010; Hume, 2008; Hume et al., 2013; Randolph and Merad, 2013), we wanted to compare brainderived CD11c-positive cells to CD11c-positive cells derived from other organs. To characterize the CD11c-positive populations of the brain, we phenotypically compared both CD45int/CD11c-positive and CD45high/CD11c-positive cells to CD11c/CD45-positive populations from spleen, liver, and lung by seven-color flow cytometry and further analyzed the influence of local factors on MHC-II expression in organotypic spleen (OSSCs) and hippocampal slice cultures (OHSCs). Materials and Methods Mice Mice strains were maintained in our animal facility at 12 h light and dark cycle and with free access to food and water. For flow cytometry analyses, 6 weeks old male wild type (wt) C57BL/6J (n 5 3–4) mice were used. For the preparation of the organotypic slice cultures, 5–8 days (d) old wt C57BL/6J mice and 5–8 d old CD11c-GFP/ DTR mice (n 5 3) (Jung et al., 2002) were used. Adult mice expressing an enhanced green fluorescent protein under the promoter of MHC-II (MHC-II-eGFP; kindly provided by Ana-Maria April 2015 Lennon-Dumenil, Institut Curie in Paris, France; n 5 4–5) (Boes et al., 2002) were used to co-cultivate isolated mononuclear cells from spleen and brain on wt organotypic slice cultures. Mice were housed in the local animal facility of the Leipzig University under the guidelines of the Animal Experimental Committee. All experiments were performed in accordance with the National Institute of Health (NIH) guidelines and approved by the local authorities. Isolation of Mononuclear Cells Animals were killed by CO2 and subsequently perfused with ice-cold PBS (Gibco, Life technologies, Darmstadt, Germany) to clear the intravascular compartment of the brain from blood cells. Brain, liver, lung, and spleen were removed and transferred into ice-cold medium (50 ml HBSS (Gibco) supplemented with 650 ml 45% D-Glucose (Sigma-Aldrich, Taufkirchen, Germany) and 750 ml 1M HEPES (Gibco). Organs were minced and homogenized using a glass potter (Novodirect, Kehl, Germany) followed by trituration of fire-polished pasteur pipettes with decreasing diameters. Cell suspension was filtered through a 70 mm cell strainer (BD Falcon, BD Biosciences, Heidelberg, Germany) followed by a careful centrifugation (Labofuge 400R, Heraeus Instruments) by 900 rpm at 4 C for 10 min. Pellets of liver, lung, and spleen cells were incubated in red-blood-cell lysis buffer for 30 min on ice and subsequently washed in PBS. Brain cells were re-suspended in 10 ml of 75% Percoll solution (GE Healthcare, M€ unchen, Germany) covered with 10 ml of 25% Percoll solution. Finally, 6 ml of PBS were carefully added on the top. The cell gradient was centrifuged at 1,800 rpm at 4 C for 30 min. After centrifugation the myelin-containing layer was carefully removed. Mononuclear brain cells were collected from the thin layer between the 25 and 75% Percoll layer. Mononuclear cells were discriminated from lymphocytes by granularity and size and for specific cell markers (see below flow cytometry staining). Preparation of OHSC Brains from 5–8 d old CD11c-DTR/GFP or wt mouse pubs were carefully removed. Forebrains were stored in ice-cold HBSS, 0.45% D-glucose, 1% PenStrep (Gibco), and 1% L-glutamine (Gibco). Further, forebrains were carefully cut in 350 mm thick slices using a vibratome (Leica VT1200S). Subsequently, hippocampi were collected and incubated for 30 min with Alexa647-conjugated isolectin B4 from Griffonia simplicifolia (IB4, 1:100; Molecular Probes, Life Technologies), diluted in pre-warmed medium containing 47% MEM (Gibco), 25% HBSS, 25% normal horse serum (Gibco), 1% glucose (45%), 1% PenStrep, and 1% L-glutamine. Slices containing hippocampus (OHSC) were washed and transferred to cell culture inserts (Millipore, Schwalbach, Germany). OHSCs from 5 to 6 d old CD11c-GFP/DTR mice were directly used for live imaging. OHSCs from 5 to 8 d old wt mice were used for co-cultivation with brain or spleen mononuclear cells and were cultivated for 6 d (37 C, 5% CO2, 60% humidity). Preparation of OSSC Spleens were removed from 5 to 8 d old mice and stored in ice-cold medium as described above. About 3% agarose gel was prepared using low melting agarose (Roth, Karlsruhe, Germany). Embedded 49 spleens were carefully cut in 350 mm thin slices with the vibratome. Subsequently, slices were collected in preparation medium and removed from agarose residues. Co-Cultivation of Brain or Spleen Mononuclear Cells on OHSC and OSSC Mononuclear cells from spleen and brain were isolated from the MHC-II-eGFP mice as described above. Isolated mononuclear cells were incubated with the fluorescent dye PKH26 (Sigma-Aldrich) dissolved in culture medium as described in the providers protocol. Of note, isolated mononuclear cells, using Percoll gradient, contain at least up to 90–97% of microglia verified by CD45int and CD11b expression. About 3 ml of the cell suspension was applied on the slice cultures and incubated for 2 h. Subsequently, live imaging was performed. In addition, slices were fixed 2, 24, and 72 h after starting co-cultivation using 1% PFA. Slices were washed with PBS and counterstained by HOECHST 33312 (Sigma). Further analyses were performed with an epifluorescence microscope (Olympus BX40) equipped with an XM10 camera. Images were processed with ImageJ software. Live Imaging Live imaging was performed using confocal microscopy (Olympus FV1000) equipped with a climate chamber at 37 C, 5% CO2, and 60% humidity. Images were acquired every 15 min for up to 24 h starting either 2, 24, or 72 h after application of MHC-II-eGFP cells. Flow Cytometry Staining Mononuclear cells were isolated and prepared as described above. Cells were pre-incubated with anti-CD16/32 antibody (1:100, eBioscience, San Diego, CA) for 10 min to minimize unspecific binding of antibodies on Fc-receptors. Next, cells were incubated for 30 min with primary labeled antibodies on ice; CD11b-Alexa Flour 700 (1:100, eBioscience), CD45-FITC (1:250, eBioscience), CD11ceFlour450 (1:80, eBioscience,), CD80-APC (1:100, eBioscience), CD86-PE-Cy5 (1:100, eBioscience), F4/80-PE-Cy7 (1:100, eBioscience), I-A[b]-PE (1:100, BD Pharmingen, BD Biosciences), or CD103-PE (1:100, BD Pharmingen). Nonprimary-labeled antibodies [CCR2 (1:200, Abcam, Cambridge, MA), CX3CR1 (1:200, Abcam), and Flt3 (1:200, Abcam) were incubated for 30 min. After washing twice with PBS, cells were incubated with the secondary antibody (goat anti-rabbit-Alexa Flour 633 (1:200, Invitrogen)]. Further, cells were rinsed in PBS and centrifuged for 2 min at 1,200 rpm. LSRII (BD) was used for cell staining measurements. For all flow cytometry stainings gates were set according to blank and isotype controls used in each experiment (Supp. Info. Fig. S1A–D). Statistical Analyses Flow cytometry stainings (percentages and MFIs) were analyzed using FlowJo (FlowJo). ImageJ was used for cell counting. Data of at least three independent experiments were evaluated by Dunnetttest or Newman-Keuls multiple comparison and presented as standard derivation 6 SD using GraphPad Prism. P values <0.05 were considered to be statistically significant (* P < 0.05, ** P 0.01, and *** P 0.001). Results Identification of Two Different CD11c/CD45Positive Cell Fractions From the Brain To phenotypically characterize the previously described population of CD11c-positive cells of the brain in direct comparison to CD11c-positive cells of other organs, we first established an isolation method valid for all investigated organs. Isolated cells were stained for CD45 with subsequent gating to CD11c and analyzed by flow cytometry (Fig. 1A). All gates were set according to the isotype controls used for all organs and stainings. The MFI of each staining was calculated by the MFI of the appropriate antibody staining subtracted by the respective isotype control. Thus, no significant differences in the percentage of CD11c-positive cells among the CD45-positive fraction were observed between brain, liver, and lung, whereas the ratio of spleen CD11-positive cells appeared to be significantly lower (Fig. 1B). According to the literature, there are two different populations of CD45-expressing cells in the brain (Carson et al., 1998; Sedgwick et al., 1991). Although CD45int cells are addressed as microglia, the CD45high fraction is represented by brain macrophages including perivascular (pv) macrophages and meningeal DCs (Fig. 1C). Of note, both brain populations contained CD11c-positive cells. In contrast, spleen, liver, and lung showed a homogenous population of CD45high-expressing cells. Further, both brain-derived CD45positive populations exhibited significantly lower amounts of CD11c-positive cells, as compared with the CD45-positive population of the lung (Fig. 1D). Of note, CD11c-positive microglia and CD11c-positive brain macrophages also showed significant lower expression levels of CD11c compared with other organs, as illustrated by the MFI (Fig. 1E). Up-Regulation of CD11c in Jv Microglia in OHSC We have previously shown that the number of CD11cpositive cells is increasing in OHSCs over time (Prodinger et al., 2011). We now performed, live imaging of IB4-labeled microglial (arrow) cells observing the up-regulation of CD11c in OHSC from 5 to 8 d old CD11c-DTR/GFP mice. We found that, migrating microglia were induced to express CD11c upon reaching microvessels (which were also demarked by IB-4 labeling, dashed line) (Fig. 2). CD11c-Positive Microglia are Lacking MHC-IIExpression MHC-II represents an important cell surface marker for DCs and APCs, relating to the ability to present antigens to CD4positive T-cells. Therefore, we in this study analyzed both CD11c-positive fractions of the brain for their expression of MHC-II in direct comparison to CD11c-positive cells from peripheral organs. In this context, both brain fractions Volume 63, No. 4 50 Immig et al: Comparison of CD11c1 Microglia FIGURE 1: Overview of the CD11c-positive cell fraction among CD45-positive cells in brain, spleen, liver, and lung in healthy mice. (A) Cells were gated in the forward scatter (FSC) for CD45. CD45-positive cells were further gated for CD11c-positive cells and (B) compared for their percentage among CD45-positive cells from brain, spleen, liver, and lung. (C) Discrimination between CD45int- and CD45high-expressing cells in the brain. (D) Comparison to the CD11c/CD45high-positive cells from spleen, liver, and lung for their percentage and MFI. April 2015 51 FIGURE 2: Up-regulation of CD11c of parenchymal and IB4-positive cells in OHSCs. OHSCs were pre-incubated with IB4 for 30 min. At regular intervals of 34 min, images of the OHSCs were acquired using confocal microscopy. The arrow marks the IB4-positive cell and the dashed line the blood vessel. showed significantly lower ratios of MHC-II-expressing cells compared with those obtained from spleen and liver (Fig. 3A). Moreover, the percentage of MHC-II expressing cells among the population of CD11c-positive microglia was dramatically lower compared with brain macrophages. In fact, the ratio of 0.47% in combination with significantly lower expression levels of MHC-II impressively indicates that MHC-II expression is almost lacking in CD11c-positive microglia (Fig. 3B). Thus, intraparenchymal CD11c-positive microglia are unique for their absence of MHC-II expression. Unique and Site-Specific Expression Patterns of F4/ 80, CX3CR1, CCR2, CD80, CD86, Flt3, and CD103 Because the above described comparison showed a unique phenotype of brain-derived CD11c/CD45-positive cells, we extended our analysis for the expression of important FIGURE 3: Comparison of MHC-II-positive cells among CD11c/CD45-positive cells in brain, liver, lung, and spleen in healthy mice. Flow cytometry analyses of the percentages (A) and MFIs (B) of MHC-II-positive cells among CD11c/CD45-positive brain cells compared with CD11c/CD45-positive cells from spleen, liver, and lung are shown. Volume 63, No. 4 52 Immig et al: Comparison of CD11c1 Microglia mononuclear and inflammatory cell markers (F4/80, CX3CR1, CCR2, CD80, CD86, Flt3, CD103) (Supp. Info. Fig. S1 and Table S1) with respect to the percentage of positive cells and intensity of protein expression measured by MFI. The highest amount of F4/80 expression was found in the CD11c-positive microglia fraction (Fig. 4A). Also, brain macrophages showed a significantly higher ratio of F4/80-positive cells compared with spleen, liver, and lung, whereas the MFI of F4/ FIGURE 4: Comparison of F4/80, CX3CR1, CD80, CD86, CCR2, CD103, Flt3-positive cells among CD11c/CD45-positive cells in brain, spleen, liver, and lung. Flow cytometry analyses of the percentages (A) and MFIs (B) of positive cells among CD11c/CD45-positive cell fractions are shown for F4/80, CX3CR1, CD80, CD86, CCR2, CD103, and Flt3. April 2015 53 FIGURE 4: (Continued) 80 in both CD11c-positive brain populations was found to be significantly lower than in spleen and liver (Fig. 4B). CD11c-positive microglia contained by far the highest percentage of CX3CR1-positive cells (Fig. 4A) with comparable MFI to mononuclear populations from other organs, despite liver macrophages (Fig. 4B). Interestingly, brain macrophages expressed only low amounts of CX3CR1 (Fig. 4A,B). The expression of the co-stimulatory molecules CD80 and CD86 measured by either percentage or MFI was similar for CD11c-positive microglia and CD11c-positive brain macrophages. However, higher amounts of CD80- and CD86positive cells were detected in the liver, whereas lung CD11cpositive cells expressed the highest MFI for CD86 (Fig. 4A). Although no significant differences were observed between the percentages of CCR2-positive cells within the CD11cpositive fractions of all isolated mononuclear cells (Fig. 4A), the CCR2 expression levels (measured by MFI) of both brain CD11c-positive fractions were significantly lower compared Volume 63, No. 4 54 Immig et al: Comparison of CD11c1 Microglia FIGURE 5: Signatures of investigating CD11c/CD45-positive cells for their expression of F4/80, CX3CR1, CD80, CD86, CCR2, CD103, and Flt3. Signatures of the (A) percentages and (B) MFI among CD11c/CD45-positive cells in brain, spleen, liver, and lung are shown for F4/80, CX3CR1, CD80, CD86, CCR2, CD103, and Flt3. with spleen, liver, and lung (Fig. 4B) and number of CCR2positive brain mononuclear cells was low under these steadystate conditions. Moreover, the highest amount of CD103positive cells was found in the CD11c-positive cell fraction of the liver. In brain macrophages, CD103 could not be detected (Fig. 4A). No differences were measured for the expression intensity of CD103-positive cells between all investigated CD11c-positive populations (Fig. 4B). Of note, CD11c-positive microglia showed the highest percentage of Flt3-positive cells (Fig. 4A). In contrast, no Flt3-positive cells were observed in the CD11c-positive brain macrophages. The highest expression intensity for Flt3 was observed in lungderived CD11c-positive cells (Fig. 4B). In summary, the comparison of crucial cell surface markers between the investigated CD11c-positive fractions of the brain parenchyma by flow cytometry revealed unique and site-specific expression patterns compared with the other extraneural CD11c-positive cells. For visualization, we combined the results to create signatures for each of the analyzed CD11c-positive cells, reflecting either the amount of positive cells (Fig. 5A) or the MFI (Fig. 5B). CD11c-Positive and Negative Microglia Show the Same Expression Patterns for MHC-II To study differences between CD11c-positive and CD11cnegative microglia, we compared the above mentioned cell markers for their percentage (Fig. 6A) and MFI (Fig. 6B). In this study, we detected a higher amount of F4/80, CCR2, April 2015 CD86, and Flt3-positive cells in the fraction of CD11cpositive microglia (Fig. 6A). Measuring the expression we observed significant higher expression of CX3CR1, but less CCR2 in CD11c-positive microglia compared with the CD11c-negative microglia (Fig. 6B). Most importantly, MHC-II expression did not differ between CD11c-positive and CD11c-negative microglia (Fig. 6A,B). Taken together, we observed that CD11c-positive microglia are not equal to CD11c-negative microglia in their immune phenotype (F4/ 80, CCR2, CD86, Flt3), but both CD11c-negative and CD11c-positive microglia shared uniquely low MHC-II levels. Microglia Maintain Their MHC-II-Negative Phenotype in the Environment of an Immune Organ To test whether the unique MHC-II-negative phenotype of microglia is driven by local cues or inherent to microglia, we transplanted microglia on OSSCs to determine whether this environment causes up-regulation of MHC-II. Vice versa, splenocytes were transplanted on entorhino-OHSCs (Hailer et al., 1998; Vinet et al., 2012) to test whether this causes down-regulation of MHC-II. As shown above, the low MHC-II expression is similar in CD11c-positive and negative microglia. Thus, we focused in this experiment on the expression of MHC-II alone, a key-molecule for antigen presentation, to investigate if the unique MHC-II-phenotype of microglia is driven by local cues or inherent. Cells were isolated from MHC-II-eGFP mice (Boes et al., 2002) and 55 FIGURE 6: Comparison of CD11c-positive and CD11c-negative microglia (CD45int). Comparison of the percentages (A) and MFIs (B) among CD11c-positive and CD11c-negative microglia for MHC-II, F4/80, CX3CR1, CCR2, CD80, CD86, CD103, and Flt3. stained using PKH26 before transplantation on slices. Using flow cytometry, we confirmed that the vast majority of brainderived mononuclear cells were MHC-II-negative, whereas splenocytes regularly expressed MHC-II (Fig. 7A). Analysis of histological sections further confirmed the absence of MHCII-positive cells in the brain parenchyma properly. However, individual MHC-II-positive cells were regularly detected in the meninges, choroid plexus and in the perivascular compartment (Fig. 7B). Upon transplantation onto organotypic slice cultures from wt mice, live imaging was performed at 2, 24, and 72 h for at least 2 h. Additional slices were fixed with 1% PFA and analyzed by fluorescence microscopy. Amoeboid PKH26-labeled splenocytes lost their eGFP expression and ramified within OHSCs (Fig. 8A,B,D), whereas brain-derived mononuclear cells remained MHC-IInegative on OSSCs (Fig. 8A,B,D). No significant changes were observed for splenocytes on OSSCs or brain mononuclear cells on OHSCs and OSSCs (Fig. 8A,B). Ramification of splenocytes on OHSCs correlated to fate of MHC-II (Fig. 8A–D). Taken together, brain mononuclear cells maintain their MHC-II-negative phenotype in the environment of an immune organ, whereas splenocytes down-regulate MHC-II and ramify to a microglia-like morphology in OHSCs. Discussion In the current study, we identified two distinct CD11cpositive cell populations within the CD45int and CD45high cell fraction of the brain under physiological conditions. CD45high- expressing cells are commonly addressed as meningeal and pv DCs, or as macrophages of peripheral origin (Carson et al., 1998; Sedgwick et al., 1991), whereas CD45int- expressing cells are commonly considered as microglia (Carson et al., 1998; Sedgwick et al., 1991), which originate from yolk sac-derived macrophage progenitors and populate the brain parenchyma during the prenatal Volume 63, No. 4 56 Immig et al: Comparison of CD11c1 Microglia FIGURE 7: Analyses of brain and spleen from MHC-II-eGFP mice. (A) Flow cytometry analyses of isolated spleen and brain mononuclear cells from MHC-II-eGFP mice labeled with PKH26. (B) Histological sections of brain and spleen from MHC-II-eGFP mice. development (Alliot et al., 1999; Ginhoux et al., 2010; Vinet et al., 2012). In previous studies Greter et al. demonstrated the existence of vessel-associated CD11c-positive cells, which appeared to be located in pv spaces (Greter et al., 2005). Furthermore, using fluorescence and electron microscopy our group identified an intraparenchymal population of CD11cpositive cells (Prodinger et al., 2011), which express the macrophage marker IBA-1 and CD11b. In continuation of our previous work, the current study demonstrates that cells expressing a microglial phenotype (CD45int, CD11b) contain a distinct subpopulation expressing CD11c. This is in line with previous studies confirming the existence of CD11cpositive cells in the embryonic, neonatal, and adult mouse brain parenchyma (Bulloch et al., 2008). However, due to the recent debate on the nature of DCs (Geissmann et al., 2010; April 2015 Hume, 2008; Randolph and Merad, 2013), the question arose whether this parenchymal population can be addressed as DCs, implying the ability to stimulate na€ıve T-cells and to transfer antigens into lymphoid organs, or whether the described population should more appropriately be addressed as a CD11c-positive fraction of microglia. In recent studies, Wlodarczyk et al. showed that CD11c-positive microglia did not express Th1- or Th17-promoting cytokines in EAE but could induce proliferation of primed CD4-positive T-cells (Wlodarczyk et al., 2014). As mentioned before, in previous studies cells expressing CD11c were addressed as macrophages, and cells addressed as DCs were shown to possess macrophage features (Bogunovic et al., 2009; Denning et al., 2007; McMahon et al., 2005; Probst et al., 2005). To avoid further confusion in the current study, the population of CD45high cells from the meninges or choroid plexus are 57 FIGURE 8: Analyses of brain and spleen mononuclear cells from MHC-II-eGFP mice, co-cultivated on wt OHSCs and OSSCs, for their MHC-eGFP expression and ramification. Comparison of MHC-II-eGFP expression (A), cell ramification (B), and the correlation (C) between MHC-II-eGFP-positive cells and ramification of brain and spleen mononuclear cells isolated from the MHC-II-eGFP mice, labeled with PKH26 and co-cultivated on wt hippocampus and spleen slice cultures. (D) Representative microscopic analyses of brain and spleen mononuclear cells isolated from the MHC-II-eGFP mouse, labeled with PKH26, are shown for the co-cultivation for the time points 2, 24, and 72 h after application on wt organotypic slice cultures from hippocampus (OHSC) and spleen (OSSC). addressed as brain macrophages. In contrast, intraparenchymal CD11c-positive cells are in this study addressed as CD11c-positive microglia, which reflects their expression of IBA-1, CD11b, and CD45int. To compare this population with CD11c-positive cells from other organs, we adapted the microglia Percoll isolation protocol (Haas et al., 2008) to isolate in the same way CD11c-positive cells from brain, spleen, liver, and lung. Volume 63, No. 4 58 Immig et al: Comparison of CD11c1 Microglia Direct comparison of the isolated CD11c-positive populations by flow cytometry showed that in contrast to CD11c-positive cells from peripheral organs, CD11c-positive microglia almost completely lacked MHC-II expression. Consistent with our observations, previous studies reported that CD11c-positive cells of the parenchyma are lacking MHC-II; its expression was found to be restricted to the meninges and choroid plexus under physiological conditions (Lv et al., 2011; Prodinger et al., 2011). In this study, we further demonstrate that the percentage of MHC-II-expressing CD11c-positive brain macrophages is also significantly lower compared with spleen and liver, which is likely to reflect the influence of local factors suppressing MHC-II expression. Thus, in contrast to the CD11c-positive cells obtained from spleen and lung, CD11c-positive microglia do not seem to be equipped for antigen-presentation to CD4-positive cells under steady state conditions. To investigate further differences in the expression for common macrophage or DC markers, we compared both CD11c-positive populations of the brain to CD11c-positive populations of peripheral organs and found that CD11cpositive microglia contain a higher percentage of cells expressing CX3CR1 and F4/80, which are known as markers for resident macrophages and microglia (Cardona et al., 2006; Carson et al., 1998; Harrison et al., 1998; Jung et al., 2000; Lewis et al., 2011; Niess, 2005; Prinz et al., 2011; Wlodarczyk et al., 2014). Further, in direct comparison to liver-, lung- and spleen-derived CD11c-positive cells, CD11cpositive microglia and brain macrophages showed significantly lower expression levels of the mononuclear cell marker CCR2 (MFI), whereas the overall percentage of CCR2-positive cells among the different CD11c-positive populations did not differ. This is in line with the view that microglia are resident, whereas CCR2-positive inflammatory monocytes are known to be highly mobile (Prinz et al., 2011). CD103-positive DCs were first identified within the lamina propria of the gut and have been described as important key players to induce immune tolerance (Scott et al., 2011). Among CD11c-positive microglia, we also detected a small amount (4.7%) of cells positive for CD103, whereas CD11-positive brain macrophages did not express CD103. In line with our data, previous studies described CD45int and CD45high CD103/CD11c double-positive cells in the olfactory bulb in a vesicular stomatitis virus model (D’Agostino et al., 2012). In this study, the CD45high expressing cells have been shown to promote T-cell proliferation, although an immunological role for the CD45int microglia-like CD103/ CD11c-positive population has not been demonstrated (D’Agostino et al., 2012). Flt3L has been shown to drive maturation to DCs in meningeal CD11c-positive cells (Anandasabapathy et al., April 2015 2011). We did not detect Flt3 in brain macrophages, but the percentage of Flt3-positive cells was highest in microglia compared with CD11c-positive cells from spleen, lung, and liver. CD80 and CD86 represent co-stimulatory molecules, which are important for antigen-presentation to T-cells. Interestingly, although MHC-II expression was not detectable in CD11c-positive microglia, the expression pattern of both costimulatory molecules turned out to be comparable to the other CD11c-positive fractions from brain, spleen, and lung under steady-state conditions. Also the MFI for both molecules was found to be similar except for CD11c-positive cells of the lung, which exhibited the highest expression intensity of CD86. Similar results have been observed in recent publications for both CD11c/CD45-positive brain populations compared with CD11c-positive cells from spleen under inflammatory conditions (Wlodarczyk et al., 2014). Further, the presence of at least one of these co-stimulatory molecules is required for T-cell activation and their expression has been described for a small subset of adult microglia (Carson et al., 1998) where CD86 seems to be related to protection and tolerance (Bechmann et al., 2001; Kuchroo et al., 1995; Mutlu et al., 2007). In the current study, we analyzed CD11c-positive cells obtained from different organs for their expression patterns of crucial DC/macrophage markers and thereby created specific signatures of their expression profiles reflecting the percentages of positive cells among the CD11c-positive fractions and the expression intensity of the investigated antigens. These signatures were strikingly different between brain, lung, liver, and spleen. Therefore, we can conclude that CD11cpositive cells represent a heterogenic population exhibiting features, which strongly depend on their origin and location. Thus, efforts for their classification or denotation in publications should be made more carefully and with respect to the mentioned parameters. Of note, comparison of CD11c-positive microglia with CD11c-negative microglia showed higher amounts of F4/80, CCR2, CD86, and Flt3-positive cells in the fraction of CD11c-positive microglia. Furthermore, CCR2 is expressed less, whereas CX3CR1 is expressed at a higher intensity in CD11c-positive microglia compared with CD11c-negative microglia. Remarkably, both microglia fractions are lacking MHC-II expression. This phenotype remained stable upon transfer onto OSSCs, whereas spleen-derived MHC-II-eGFP cells lost their green fluorescence and ramified on OHSCs supporting the view that local factors specifically adopt mononuclear cells within the brain’s parenchyma (Abutbul et al., 2012; Hailer et al., 1998) where this ramified, “inactivated” phenotype exhibits neuroprotective effects (Abutbul et al., 2012). Our data provide an overview of the phenotype of different CD11c-positive cells derived from different organs and 59 specifically demonstrate the unique and “inactivated” phenotype of CD11c-positive microglia under physiological conditions. These findings are useful in studying changes of brain CD11c-positive populations under neuroinflammatory conditions in comparison to changes of CD11c-positive cells derived from other organs. This could give new insights for the understanding of the progression and development of chronic neuroinflammatory diseases such as MS and Alzheimer‘s disease. Randolph GJ, Merad M. 2009. Origin of the lamina propria dendritic cell network. Immunity 31:513–525. Bulloch K, Miller MM, Gal-Toth J, Milner TA, Gottfried-Blackmore A, Waters EM, Kaunzner UW, Liu K, Lindquist R, Nussenzweig MC, Steinman RM, McEwen BS. 2008. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J Comp Neurol 508:687–710. Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. 2006. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9:917–924. Carson MJ, Reilly CR, Sutcliffe JG, Lo D. 1998. Mature microglia resemble immature antigen-presenting cells. Glia 22:72–85. Acknowledgment Grant sponsor: Deutsche Forschungsgemeinschaft; Grant number: DFG-FOR1336 (B2 to IB, C1 to KB, and A1 to UKH); Grant sponsor: Helmholtz Alliance ICEMED (Imaging and Curing Environmental Metabolic Diseases). The authors thank Sonja Kallendrusch (Institute of Anatomy, Leipzig University) and Kristin Schubert (Department of Experimental Rheumatology, University Hospital Leipzig) for the help in establishing the co-cultivation experiment. Further the authors thank to Lena Wendeburg (Department of Psychiatry and Psychotherapy, Section of Molecular Psychiatry, University of Freiburg, Freiburg) for the help in establishing the isolation of mononuclear cells and Constanze Hobusch (Institute of Anatomy, Leipzig University) and Bianka Graf (Institute of Anatomy, Leipzig University) for technical assistance in tissue preparation. Also the authors thank to Dr. Petra FinkSterba, Dr. Petra Hirrlinger, and Sigrid Weisheit (MedizinischExperimentelles Zentrum, Leipzig University) for animal care. D’Agostino PM, Kwak C, Vecchiarelli HA, Toth JG, Miller JM, Masheeb Z, McEwen BS, Bulloch K. 2012. Viral-induced encephalitis initiates distinct and functional CD1031 CD11b1 brain dendritic cell populations within the olfactory bulb. Proc Natl Acad Sci USA 109:6175–6180. Denning TL, Wang Y-C, Patel SR, Williams IR, Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 8:1086–1094. Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. 2010. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10:453–460. Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845. Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. 2006. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukocyte Biol 80:797–801. Gottfried-Blackmore A, Kaunzner UW, Idoyaga J, Felger JC, McEwen BS, Bulloch K. 2009. Acute in vivo exposure to interferon- enables resident brain dendritic cells to become effective antigen presenting cells. Proc Natl Acad Sci USA 106:20918–20923. References Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. 2005. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11:328–334. Abutbul S, Shapiro J, Szaingurten-Solodkin I, Levy N, Carmy Y, Baron R, Jung S, Monsonego A. 2012. TGF-b signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia 60:1160– 1171. Haas AHD, Boddeke HWGM, Biber K. 2008. Region-specific expression of immunoregulatory proteins on microglia in the healthy CNS. Glia 56:888–894. Alliot F, Godin I, Pessac B. 1999. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117:145–152. Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. 2011. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med 208:1695–1705. Bechmann I, Goldmann J, Kovac AD, Kwidzinski E, Simb€ urger E, Naftolin F, Dirnagl U, Nitsch R, Priller J. 2005. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. FASEB J 19:647–649. Bechmann I, Peter S, Beyer M, Gimsa U, Nitsch R. 2001. Presence of B7–2 (CD86) and lack of B7–1 (CD(80) on myelin phagocytosing MHC-II-positive rat microglia is associated with nondestructive immunity in vivo. FASEB J 15: 1086–1088. Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen J, Ploegh HL. 2002. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature 418:983–988. Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Hailer NP, Heppner FL, Haas D, Nitsch R. 1998. Astrocytic factors deactivate antigen presenting cells that invade the central nervous system. Brain Pathol 8:459–474. Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. 1998. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA 95:10896–10901. Hume DA. 2008. Macrophages as APC and the dendritic cell myth. J Immunol 181:5829–5835. Hume DA, Mabbott N, Raza S, Freeman TC. 2013. Can DCs be distinguished from macrophages by molecular signatures? Nat Immunol 14:187–189. Ji Q, Castelli L, Goverman JM. 2013. MHC class I–restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD81 T cells. Nat Immunol 14:254–261. Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20:4106–4114. Jung S, Unutmaz D, Wong P, Sano G-I, los Santos Kd, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Parner EG, Littman DR, Lang RA. 2002. In vivo depletion of CD11c1 dendritic cells abrogates priming of CD81 T cells by exogenous cell-associated antigens. Immunity 17:211–220. Volume 63, No. 4 60 Immig et al: Comparison of CD11c1 Microglia Kaminski M, Bechmann I, Kiwit J, Glumm J. 2012. Migration of monocytes after intracerebral injection. Cell Adh Migr 6:164–167. Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, H€ olscher C, M€ uller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. 2013. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16:273–280. Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: Application to autoimmune disease therapy. Cell 80:707–718. Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. 2011. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity 35:780–791. Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. 2004. Visualizing dendritic cell networks in vivo. Nat Immunol 5:1243–1250. Locatelli G, W€ ortge S, Buch T, Ingold B, Frommer F, Sobottka B, Kr€ uger M, Karram K, B€ uhlmann C, Bechmann I, Heppner FL, Waisman A, Becher B. 2012. Primary oligodendrocyte death does not elicit anti-CNS immunity. Nature Neuroscience 15:543–550. Nakamoto N, Ebinuma H, Kanai T, Chu P-S, Ono Y, Mikami Y, Ojiro K, Lipp M, Love PE, Saito H, Hibi T. 2012. CCR91 macrophages are required for acute liver inflammation in mouse models of hepatitis. Gastroenterology 142:366–376. Niess JH, Brand S, Gu X, Landsmann L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littmann DR, Reinecker HC. 2005. CX3CR1mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254–258. Prinz M, Priller J. 2010. Tickets to the brain: Role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J Neuroimmunol 224:80–84. Prinz M, Priller J, Sisodia SS, Ransohoff RM. 2011. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 13:1227–1235. Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, van Den Broek M. 2005. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol 141:398–404. Prodinger C, Bunse J, Kr€ uger M, Schiefenh€ ovel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I. 2011. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol 121:445–458. Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L. 2008. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA 105:19869–19874. Randolph G, Merad M. 2013. Reply to: "Can DCs be distinguished from macrophages by molecular signatures?". Nat Immunol 14:189–190. Lv M, Liu Y, Zhang J, Sun L, Liu Z, Zhang S, Wang B, Su D, Su Z. 2011. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience 176:162–172. Scott CL, Aumeunier AM, Mowat AM. 2011. Intestinal CD1031 dendritic cells: Master regulators of tolerance? Trends Immunol 32:412–419. Matyszak MK, Perry VH. 1996. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience 74:599–608. Sedgwick JD, Schwender S, Imrich H, D€ orries R, Butcher GW, ter Meulen V. 1991. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA 88:7438–7442. McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. 2005. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med 11:335–339. Steinman RM, Cohn ZA. 2007. Pillars article: Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973. 137: 1142–1162. J Immunol 178:5–25. McMenamin PG. 1999. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol 405:553–562. Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. 2003a. Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. Ann NY Acad Sci 987:15–25. McMenamin PG, Wealthall RJ, Deverall M, Cooper SJ, Griffin B. 2003. Macrophages and dendritic cells in the rat meninges and choroid plexus: Threedimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res 313:259–269. Steinman RM, Hawiger D, Nussenzweig MC. 2003b. Tolerogenic dendritic cells. Annu Rev Immunol 21:685–711. Mesnil C, Sabatel CM, Marichal T, Toussaint M, Cataldo D, Drion P-V, Lekeux P, Bureau F, Desmet CJ, Ryffel B. 2012. Resident CD11b1Ly6C2 lung dendritic cells are responsible for allergic airway sensitization to house dust mite in mice. PLoS One 7:e53242. Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch U-K, Mack M, Heikenwalder M, Br€ uck W, Priller J, Prinz M. 2007. Microglia in the adult brain arise from Ly-6ChiCCR21 monocytes only under defined host conditions. Nat Neurosci 10:1544–1553. Mohammad MG, Hassanpour M, Tsai VWW, Li H, Ruitenberg MJ, Booth DW, Serrats J, Hart PH, Symonds GP, Sawchenko PE, Breit SN, Brown DA. 2012. Dendritic cells and multiple sclerosis: Disease, tolerance, and therapy. Int J Mol Sci 14:547–562. Mutlu L, Brandt C, Kwidzinski E, Sawitzki B, Gimsa U, Mahlo J, Aktas O, Nitsch R, Zwam M, Laman JD, Bechmann I. 2007. Tolerogenic effect of fiber tract injury: reduced EAE severity following entorhinal cortex lesion. Exp Brain Res 178:542–553. April 2015 van Rijt LS. 2005. In vivo depletion of lung CD11c1 dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 201:981–991. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. 2005. In vivo depletion of lung CD11c1 dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 201:981–991. van Zwam M, Huizinga R, Melief M-J, Wierenga-Wolf AF, Meurs M, Voerman JS, Biber KPH, Boddeke HWGM, H€ opken UE, Meisel C, Meisel A, Bechmann I, Hintzen RQ, ’t Hart BA, Amor S, Laman JD, Boven LA. 2009. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med 87:273–286. Vinet J, van Weering HRJ, Heinrich A, K€ alin RE, Wegner A, Brouwer N, Heppner FL, van Rooijen N, Boddeke HW, Biber K. 2012. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflamm 9:27. Wlodarczyk A, Løbner M, C edile O, Owens T. 2014. Comparison of microglia and infiltrating CD11c1 cells as antigen presenting cells for T cell proliferation and cytokine response. J Neuroinflamm 11:57. 61 5 Zusammenfassung Dissertation zur Erlangung des akademischen Grades Dr. rer. med. Titel: Immunphänotypische Charakterisierung CD11c-positiver Zellen des Gehirns im direkten Vergleich zu CD11c-positiven Zellen von Lunge, Leber und Milz Eingereicht von: Kerstin Immig Angefertigt am: Institut für Anatomie der Medizinischen Fakultät der Universität Leipzig Betreuer: Herr Professor Dr. med. Ingo Bechmann Eingereicht im: 21.07.2015 Das Fehlen von Dendritischen Zellen (DCs) im Gehirn wurde oft in kausalen Zusammenhang zum Immunprivileg des Gehirns gebracht. Die Ansicht, dass es keine DCs im Gehirn gibt, wurde jedoch mit der Beobachtung von CD11c- und Major Histocompatibility Complex (MHC)-II-positiven Zellen in den Meningen und im Plexus choroideus sowohl in Ratten als auch in Mäusen in Frage gestellt. CD11c und MHC-II sind zwei Marker, welche am häufigsten für die Identifizierung von DCs verwendet wurden. Da aber fast alle zur Verfügung stehenden Antikörper gegen DCs unter nichtinflammatorischen Bedingungen keine klaren Ergebnisse im neuralen Gewebe erbrachten, gab es kaum Untersuchungen von DCs unter diesen Bedingungen. Die Entwicklung des transgenen Mausmodells, welche unter dem Promotor von CD11c das grün-fluoreszierende Protein (GFP) exprimiert, war ein entscheidender Schritt, DCs und CD11c-positive Zellen unter nichtinflammatorischen Bedingungen im Gehirn zu untersuchen. Unter Benutzung dieses Mausmodells untersuchten wir CD11c-positive Zellen aus dem Gehirn unter gesunden Bedingungen und verglichen diese mit CD11c-positiven Zellen aus anderen Organen. Dabei zeigten wir zum ersten Mal, dass auch im gesunden Mausgehirn juxtavaskuläre, intraparenchymal lokalisierte Zellen existieren, welche CD11c exprimieren. Darüber hinaus waren die Zellen in der Tela des Plexus choroideus, unter dem Ependym der lateralen Ventrikel, entlang des Corpus callosum und um den Fornix verteilt. Im Gegensatz zu den meisten im Plexus choroideus lokalisierten CD11c-positiven Zellen, zeigten wir, dass die intraparenchymal lokalisierten Zellen ebenfalls die Mikroglia-/Makrophagenmarker IBA-1 und CD11b exprimierten. Auch vereinzelte Fortsätze dieser CD11c-positiven Zellen waren am Aufbau der Glia limitans beteiligt. Sowohl mittels konfokaler, als auch mittels 62 Elektronenmikroskopie, beobachteten wir, dass diese Fortsätze an der Bildung der Glia limitans beteiligt waren und bis an ihre Basalmembran grenzten. Weitergehend nutzten wir das Modell der organotypischen Schnittkulturen des Hippocampus (OHSC) und zeigten, dass CD11c-positive Zellen sich aus intraparenchymalen Vorläufern entwickeln können. Ebenso wiesen wir nach, dass sich die CD11c-positiven Zellen auch aus Vorläuferzellen des Knochenmarks entwickeln können. Zusammenfassend konnten wir zeigen, dass intraparenchymal lokalisierte Zellen Eigenschaften von DCs teilen, in dem sie CD11c exprimieren, aber zeitgleich auch typische Expressionsmarker für Mikroglia bzw. Makrophagen aufwiesen. Etwa zur gleichen Zeit wurde in zahlreichen Publikationen gezeigt, dass der DC-Marker CD11c auch auf anderen Zelltypen, etwa wie B-Zellen und Makrophagen, exprimiert werden kann. Dies führte zu der Diskussion, ob sich DCs als eigenständige Zellpopulation darstellen lassen und ob sie überhaupt von Makrophagen unterschieden werden können. Deshalb stellte sich uns die Frage wie sich CD11c-exprimierende Zellen des Gehirns von CD11cpositiven Zellen aus anderen Organen unterscheiden. Dafür isolierten wir mit Hilfe eines einheitlichen Isolierungsprotokolls mononukläre Zellen aus dem Gehirn sowie aus der Lunge, der Leber und der Milz. Danach färbten wir diese Zellen mit etablierten monozytären und inflammatorischen Zellmarkern und verglichen diese Zellen miteinander mittels Durchflusszytometrie. Dabei nutzten wir den prozentualen Anteil der positiven Zellen für den entsprechenden Marker als auch die Expressionsintensität (MFI), welche eine Aussage erlaubt, wie stark der entsprechende Marker von der Zelle exprimiert wird. Zunächst wurden die isolierten Zellen auf den Panleukozytenmarker CD45 untersucht. In der Lunge, Leber und Milz haben wir eine homogene CD45 hoch-exprimierende (CD45high) Population isolieren können, wohingegen im Gehirn hinsichtlich der Expression von CD45 zwei unterschiedliche Populationen nachweisbar waren. Wie bereits in der Literatur beschrieben, ist die CD45intermediär (CD45int)-exprimierende Population, welche einen Anteil von bis zu 99 % der mononukleären Zellen im Gehirn ausmacht, als Mikroglia beschrieben. Bei den wenigen CD45high-exprimierenden Zellen handelt es sich wiederum um Makrophagen und DCs aus den Meningen und dem Plexus choroideus. Danach haben wir unsere isolierten Zellen auf die Expression von CD11c untersucht und konnten in allen Populationen CD11c-positive Zellen isolieren. Der prozentuale Anteil CD11c-positiver Zellen in der Mikrogliapopulation war geringer als der prozentuale Anteil in der Lunge und Leber. Auch der prozentuale Anteil CD11c-positiver Zellen unter den perivaskulären Makrophagen/DCs war geringer als jener in der Lunge. Weiterhin untersuchten wir die isolierten Populationen auf wichtige inflammatorische und mononukleäre Zellmarker (CX3CR1, F4/80, CD80, CD86, MHC-II, CCR2, CD103) und identifizierten einzigartige und umgebungsspezifische Eigenschaften dieser Zellen. So exprimierten die CD11c-positiven 63 Mikroglia nicht nur den DC-Marker CD11c, auch der Anteil an typischen Mikrogliamarkern wie CX3CR1 und F4/80 war am höchsten im Vergleich zu CD11c-positiven Zellen aus Lunge, Leber und Milz. Im Gegensatz dazu waren beide CD11c-positiven Gehirnpopulationen nahezu negativ in der Expressionsintensität für den inflammatorischen Monozytenmarker CCR2. Durch die Zusammenfassung aller Marker haben wir spezifische Signaturen erstellt, welche die Heterogenität jeder untersuchten CD11c-positiven Population in Gehirn, Milz, Lunge und Leber verdeutlichen. Beim Vergleich der CD11c-positiven Populationen für MHC-II, einem wichtigen Protein für die Antigenpräsentation, stellten wir fest, dass beide Gehirnpopulationen im Vergleich zur Milz und Leber einen geringeren Anteil an MHC-II-positiven Zellen beinhalteten. Darüber hinaus war die Expressionsintensität von MHC-II in den CD11c-positiven Mikroglia fast nicht messbar. Schlussfolgernd lässt sich daraus ableiten, dass die Mikroglia einzigartig in ihrer Expression für MHC-II im Vergleich zu den anderen untersuchten CD11c-positiven Populationen aus Lunge, Leber und Milz sind. Nachdem wir zeigen konnten, dass die CD11c-positiven Mikroglia in ihrer MHC-II-Expression einzigartig sind und sich zudem in dieser Eigenschaft nicht von den CD11c-negativen Mikroglia unterschieden, wollten wir anhand dieses Beispiels untersuchen, ob diese Eigenschaft zellspezifisch ist oder durch die Umgebung im Gehirn beeinflusst wird. Dafür haben wir isolierte mononukleäre Zellen von einer Mauslinie, welche unter dem Promotor von MHC-II das verstärkte grün-fluoreszierende Protein (eGFP) exprimiert, auf Schnittkulturen von Hippocampus (OHSC) und Milz (OSSC) von Wildtypmäusen (Wt) cokultiviert. Durch die Benutzung eines konfokalen Mikroskops und eines Live-Imaging Systems konnten wir die Schnittkulturen, co-kultiviert mit den Einzelzellen, über einen Zeitraum von mindestens 72 h beobachten. Mit Hilfe des rot-fluoreszierenden Farbstoffes PKH26 konnten wir die transplantierten MHC-II-negativen Zellen aus der MHC-II-eGFPMaus von den Wt-Zellen unterscheiden. Hauptsächlich waren die Mikroglia MHC-II-negativ und Milzzellen MHC-II-positiv. Während unserer Beobachtungen sahen wir, dass sich Milzzellen begannen zu ramifizieren, wenn sie auf OHSCs kultiviert wurden, wobei zeitgleich die Expression von MHC-II deutlich geringer wurde. Wenn diese Zellen hingegen auf OSSCs co-kultiviert wurden, blieben diese hauptsächlich MHC-II-positiv und behielten ihre amöboide Form. Weiterhin blieben Mikroglia, co-kultiviert auf OSSCs, MHC-II-negativ und ebenfalls amöboid. Mikroglia, kultiviert auf OHSCs, ramifizierten dagegen und blieben MHC-II-negativ. Dies ließ uns schlussfolgern, dass immunologisch aktive Zellen aus der Peripherie durch Umgebungsfaktoren im Gehirn beeinflusst werden und unter nichtinflammatorischen Bedingungen einen eher antiinflammatorischen Phänotyp entwickeln. Darüber hinaus behalten Mikroglia außerhalb ihrer Umgebung, in diesem Fall in der Umgebung eines Immunorgans, unter nichtinflammatorischen Bedingungen, ihren inaktiven Phänotyp. 64 Diese Daten unterstützen die Ansicht, dass lokale Faktoren mononukleäre Zellen beeinflussen und nichtinflammatorische Bedingungen schaffen, um im immunprivilegierten Gehirnparenchym die neuroprotektiven Effekte aufrecht zu erhalten. Darüber hinaus konnten wir in unserem Modell zeigen, dass Mikroglia, unter nichtinflammatorischen Bedingungen, auch in der Umgebung eines Immunorgans ihren intrinsischen Phänotyp behalten können. Diese Ergebnisse charakterisieren CD11c-positive Zellen des Gehirns als eigenständigen Typus, den wir als Signatur dargestellt haben. Interessanterweise zeigen sich solche aber auch für CD11c-positive Zellen aus anderen Organen, sodass von einer ubiquitär stattfindenden lokalen Adaption ausgegangen werden kann. Die Festlegung der Signaturen von CD11c-positiven und CD11c-negativen Mikroglia erlaubt uns die Untersuchung von Veränderungen in verschiedenen Pathologien wie der Multiplen Sklerose und der Alzheimerschen Erkrankung. Aufbauend auf den vorhergehenden Ergebnissen möchten wir nun herausfinden, ob Mikroglia eine wichtige Rolle in Pathologien der Adipositas spielen können. Dafür untersuchen wir Mäuse, welche eine spezifische fettreiche oder kohlenhydratreiche Futterdiät erhalten haben. Da der Hypothalamus ein wichtiges Kontrollzentrum für Hunger ist, wollen wir Mikroglia aus dem Hypothalamus mit Mikroglia aus dem Cortex immunphänotypisch miteinander vergleichen. Erste Ergebnisse deuten auf ein verändertes Phagozytoseverhalten von Mikroglia hin. . 65 6 Summary One of the concepts for the immune privilege of the brain has been attributed to the lack of Dendritic Cells (DCs). This view became challenged by the identification of CD11c and Major Histocompatibility Complex (MHC)-II-positive cells in the meninges and choroid plexus in the brain of healthy mice and rats. These two proteins are the most prominent markers used for the identification of DCs. However, under non-inflammatory conditions available antibodies only provided questionable results when applied on central nervous system (CNS) tissue. The development of the transgenic mouse model in which the green fluorescent protein (GFP) is expressed under the promoter of CD11c (itgax) allowed the investigation of CD11cpositive cells under non-inflammatory conditions in the brain. Using the advantage of this mouse model and flow cytometry analyzes, we have investigated CD11c-positive cells in the brain parenchyma and compared these cells to CD11c-positive cells derived from other organs. In our studies we have demonstrated the existence of intraparenchymal CD11c-positive cells in the brain of healthy mice for the first time. Our observations showed that these cells are distributed in the tela of the choroid plexus, below the ependyma of the lateral ventricle, along the corpus callosum and within the fornix. Beside the DC-marker CD11c, the cells located within the parenchyma also expressed typical macrophage and microglia markers such as IBA-1 and CD11b. Additionally, using electron and confocal microscopy, we identified that some processes of the intraparenchymal CD11c-positive cells take part in the formation of the glia limitans. Moreover, using organotypic hippocampal slice cultures (OHSCs) we observed that these CD11c-positive cells can be derived from intrinsic precursors as well as from the bone-marrow using a chimeric mouse model. To summarize, we identified and characterized intraparenchymal cells, expressing the DC marker CD11c as well as the macrophage/microglia marker IBA-1 and CD11b. The demonstration that the DC-Marker CD11c can also be expressed from macrophages or B-cells, gave rise to a discussion about the existence of DCs as an independent cell line and if DCs can in fact be clearly distinguished from macrophages. Therefore, we addressed the question on how brain-derived CD11c-positive cells differ from CD11c-positive cells derived from other organs. Here, we used acutely isolated mononuclear cells from brain, spleen, liver and lung and analyzed them for the expression of important mononuclear and inflammatory cell markers using flow cytometry. We labeled the isolated cells for the pan leukocyte marker CD45 and observed two different CD45-expressing cells in the brain, whereas the other organs exhibited a more homogenous CD45 high (CD45high) expressing population. As it is described in the literature the CD45 intermediate (CD45int) expressing population is known as 66 microglia and the CD45high expressing population is defined as perivascular or meningeal macrophages and DCs. From all investigated CD45-positive populations we isolated CD11cpositive cells. Here, the percentage of CD11c-positive cells among the microglia fraction was significantly lower compared to that of lung and liver. Further, the percentage of CD11cpositive cells among the perivascular macrophages and DCs was significantly lower compared to the lung. Moreover, comparing cells for other important inflammatory and mononuclear cell markers (CX3CR1, F4/80, CD80, CD86, MHC-II, CCR2, CD103), we found unique and site-specific expression patterns for each organ. The fraction of CD11c-positive microglia contained the highest amount of CX3CR1 and F4/80-positive cells compared to the other investigated CD11c-positive cell populations. In contrast, both CD11c-positive brainderived populations almost completely lacked the inflammatory monocyte marker CCR2. Additionally, to demonstrate the heterogeneity of CD11c-positive populations from brain, spleen, liver and lung we summarized all investigated cell markers in specific signatures. Comparing the expression of MHC-II, an important cell marker for antigen presentation to Tcells, we observed that the amount of MHC-II-positive cells among both CD11c-positive brain populations was significantly lower compared to spleen and liver. Investigating the expression intensity of MHC-II, we found that CD11c-positive microglia almost completely lacked the MHC-II expression. These results indicate that CD11c-positive microglia are unique for their low MHC-II expression in comparison to the other investigated CD11cpositive populations. Besides, we did not find any differences between the MHC-II expression of CD11c-positive microglia and CD11c-negative microglia. To test whether phenotypical differences of microglia are fixed by origin or developed due to environmental factors, we established the co-cultivation of acutely isolated mononuclear cells from spleen and brain from the MHC-II-enhanced green fluorescent protein (eGFP) mice on organotypic slice cultures from hippocampus (OHSC) and spleen (OSSC) from wild type (wt) mice. Using confocal microscopy, we established a live-imaging set-up to observe our cocultivated single cells on wt slices over at least 72 h. To differentiate MHC-II-negative cells isolated from the MHC-II-eGFP mouse, from wt cells, we used the red fluorescent dye PKH26. Here, we observed that spleen cells co-cultivated on OHSCs mainly reduced their MHC-II-expression, which was paralleled by enhanced ramification. In contrast, mononuclear cells obtained from spleen did not reduce their MHC-II-expression nor ramified on OSSCs. Investigating brain mononuclear cells co-cultivated on OSSCs, we observed no up-regulation of MHC-II or any ramification. Brain mononuclear cells co-cultivated on OHSCs remained MHC-II-negative but expressed a ramified morphology. In summary, peripheral cells adapt to the environment of the brain and transform to an immunological inactivated phenotype while microglia keep their immunological inactivated phenotype in the environment of an immune organ under non-inflammatory conditions. 67 These investigations support the view, that local factors influence mononuclear cells and inactivate pro-inflammatory features under physiological conditions to maintain the neuroprotective effects of the brain`s immune privilege. Moreover, microglia show an intrinsic phenotype, which does not change in the environment of an immune organ under noninflammatory conditions. These findings characterize the intraparenchymally located CD11c-positive brain population as an independent cell type which we illustrated in specific signatures. Interestingly, such specific signatures can be displayed for other DCs derived from other organs. Therefore we suppose that beside a local adaption of cells in the brain, also a ubiquitary adaption to the local environment occurs. The definition of specific signatures of CD11c-positive and negative microglia may help to investigate changes in different pathologies such as multiple sclerosis and Alzheimer`s disease. In continuation to our previous work we would like to invest if microglia may have an important role in different pathologies of obesity. Therefore, we now investigate mice under high fat or high carbohydrate diet in comparison to mice under chow diet. Since the hypothalamus is an important brain region to regulate hunger we want to isolate microglia from the hypothalamus and from the cortex to compare the immunological phenotype. Preliminary results indicate a different behavior of phagocytosis of microglia in mice which received a high caloric diet. 68 7 Danksagung Die vorliegende Promotionsarbeit entstand am Institut für Anatomie der Universität Leipzig. Hiermit möchte ich mich für die Überlassung des Themas herzlichst bei meinem Doktorvater Prof. Dr. med. Ingo Bechmann bedanken. Durch seine stete Betreuung, seine Ermutigung zum selbständigen wissenschaftlichen Denken und sein vorgelebter Enthusiasmus an der Wissenschaft, erlaubte mir große Freude an dieser Arbeit zu entwickeln. Ein großes Dankeschön möchte ich auch an alle Mitarbeiter des Instituts für Anatomie und den Mitgliedern unserer Arbeitsgruppe, besonders an Dr. med. Martin Gericke, Dr. med. Martin Krüger, Dr. rer. med. Felicitas Merz, Franziska Menzel, Julia Landmann und PD. Dr. phil. nat. Marco Koch für die Geduld und Unterstützung während der Doktorarbeit aussprechen. Weiterhin möchte ich mich bei den Mitarbeitern der IZKF-FACS Core Unit bedanken. Zusätzlich geht ein großes Dankeschön an alle Mitglieder der Forschergruppe DFG 1336 für die tolle Organisation und den jährlichen Retreats, welche das Thema, durch kritische Diskussionen, in großen Schritten vorangetrieben haben. Meinen größten Dank möchte ich an meine Familie richten, welche mich mein ganzes Leben unterstützt hat und mir immer Mut und Zuversicht zugesprochen hat. Zum Schluss geht noch ein großes Dankeschön an alle meine Freunde, welche mich auf meinem bisherigen Weg begleitet haben und mich unterstützen. Dankeschön! 69 8 Erklärung über die eigenständige Abfassung der Arbeit Hiermit erkläre ich, dass ich die vorliegende Arbeit selbständig und ohne unzulässige Hilfe oder Benutzung anderer als der angegebenen Hilfsmittel angefertigt habe. Ich versichere, dass Dritte von mir weder unmittelbar noch mittelbar geldwerte Leistungen für Arbeiten erhalten haben, die im Zusammenhang mit dem Inhalt der vorgelegten Dissertation stehen, und dass die vorgelegte Arbeit weder im Inland noch im Ausland in gleicher oder ähnlicher Form einer anderen Prüfungsbehörde zum Zweck einer Promotion oder eines anderen Prüfungsverfahrens vorgelegt wurde. Alles aus anderen Quellen und von anderen Personen übernommene Material, das in der Arbeit verwendet wurde oder auf das direkt Bezug genommen wird, wurde als solches kenntlich gemacht. Insbesondere wurden alle Personen genannt, die direkt an der Entstehung der vorliegenden Arbeit beteiligt waren. ................................. Datum .................................... Unterschrift 70 9 Curriculum vitae Name: Kerstin Immig Anschrift: Bernhard-Göring-Straße 91 04275 Leipzig Geburtsort: 11.08.1983, Leipzig Familienstand: ledig Schulbildung: 1990-1994 Heinrich-Pestalozzi-Schule (Grundschule) 1994-1999 Uhlandschule (Gymnasium) 1999-2002 Max-Klinger-Schule (Gymnasium) 2002: Abitur Studium: 2002-2004 Studium der Biologie (Grundstudium), Universität Leipzig 2003-2004 ERASMUS an der Neuen Bulgarischen Universität Sofia 2004-2008 Studium der Biologie (Diplom), Universität Rostock Promotion: seit: 2010 an der Universität Leipzig Thema: Immunphänotypische Charakterisierung CD11c-positiver Zellen des Gehirns im direkten Vergleich zu CD11c-positiven Zellen von Lunge, Leber und Milz Preise: 2011- 10. Research Festival der Universität Leipzig, Posterpreis 2012- 11. Research Festival der Universität Leipzig, Posterpreis Berufserfahrung: - Oktober 2008 bis Mai 2010 wissenschaftliche Mitarbeiterin am Institut für allgemeine Pharmakologie der Johann-Wolfgang Goethe Universität Frankfurt - seit Mai 2010 wissenschaftliche Mitarbeiterin am Institut für Anatomie der Universität Leipzig 71 Publikationen: Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I (2010) CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 121:445-58 Fischer W, Urban N, Immig K, Franke H, Schaefer M (2013) Natural compounds with P2X7 receptor-modulating properties. Purinergic Signal. 10:313-26 Haase J, Weyer U, Immig K, Klöting N, Blüher M, Eilers J, Bechmann I, Gericke M (2013) Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 57:562-71 Preissler J, Grosche A, Lede V, Le Duc D, Krügel K, Matyash V, Szulzewsky F, Kallendrusch S, Immig K, Kettenmann H, Bechmann I, Schöneberg T, Schulz A (2014) Altered microglial phagocytosis in GPR34-deficient mice. Glia 63(2):206-15 Krueger M, Bechmann I, Immig K, Reichenbach A, Härtig W, Michalski D (2015) Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 35(2):292-30. Immig K, Gericke M, Menzel F, Merz F, Krueger M, Schiefenhövel F, Lösche A, Jäger K, Hanisch UK, Biber K, Bechmann I (2015) CD11c-positive cells from brain, spleen, lung, and liver exhibit site-specific immune phenotypes and plastically adapt to new environments. Glia. 63(4):611-25.